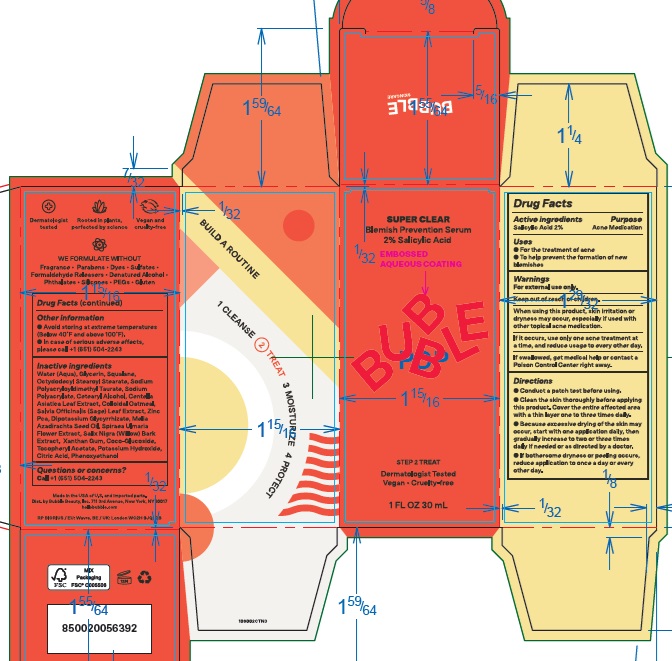
Label: BUBBLE SUPER CLEAR BLEMISH PREVENTION SERUM- salicylic acid emulsion
- NDC Code(s): 83509-001-01
- Packager: BUBBLE BEAUTY
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Conduct a patch test before using.
- Clean the skin thoroughly before applying this product. Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
-
Inactive ingredients
Water (Aqua), Glycerin, Squalane, Octyldodecyl Stearoyl Stearate, Sodium Polyacryloyldimethyl Taurate, Sodium Polyacrylate, Cetearyl Alcohol, Centella Asiatica Leaf Extract, Colloidal Oatmeal, Salvia Officinalis (Sage) Leaf Extract, Zinc Pca, Dipotassium Glycyrrhizate, Melia Azadirachta Seed Oil, Spiraea Ulmaria Flower Extract, Salix Nigra (Willow) Bark Extract, Xanthan Gum, Coco-Glucoside, Tocopheryl Acetate, Potassium Hydroxide, Citric Acid, Phenoxyethanol
- Questions or concerns?
- Product Packaging
-
INGREDIENTS AND APPEARANCE
BUBBLE SUPER CLEAR BLEMISH PREVENTION SERUM
salicylic acid emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83509-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) OATMEAL (UNII: 8PI54V663Y) ZINC PIDOLATE (UNII: C32PQ86DH4) SALIX NIGRA BARK (UNII: QU52J3A5B3) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FILIPENDULA ULMARIA FLOWER (UNII: 06L18L32G6) XANTHAN GUM (UNII: TTV12P4NEE) COCO-GLUCOSIDE (UNII: ICS790225B) SAGE (UNII: 065C5D077J) AZADIRACHTA INDICA SEED OIL (UNII: 4DKJ9B3K2T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM POLYACRYLOYLDIMETHYL TAURATE (UNII: NG5NG5733T) WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83509-001-01 1 in 1 CARTON 07/01/2023 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/01/2023 Labeler - BUBBLE BEAUTY (117396016)