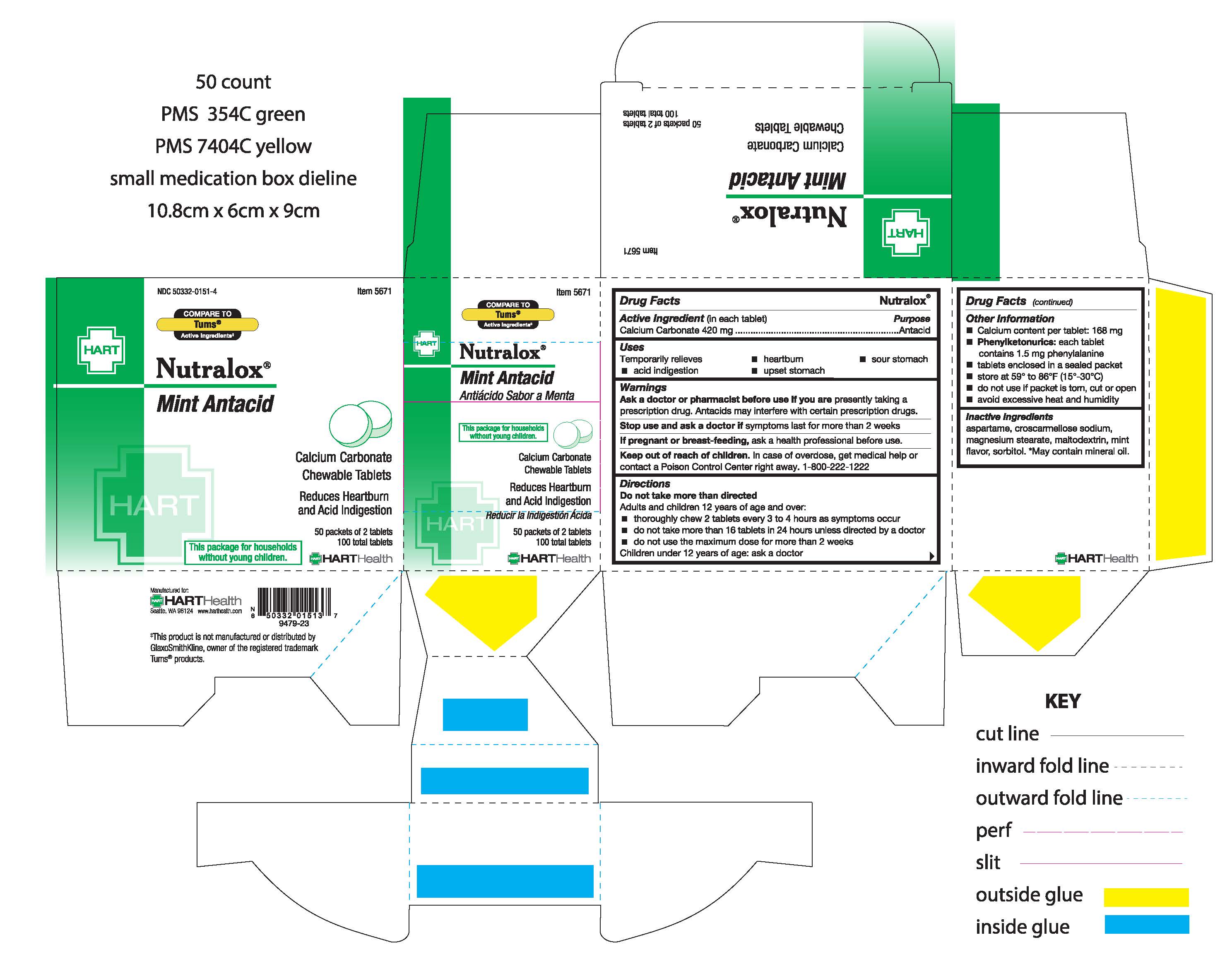
Label: NUTRALOX- calcium carbonate tablet, chewable
-
NDC Code(s):
50332-0151-1,
50332-0151-3,
50332-0151-4,
50332-0151-7, view more50332-0151-8
- Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 9, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
Do not take more than directed
Adults and children 12 years of age and over:
- thoroughly chew 2 tablets every 3 to 4 hours as symptoms occur
- do not take more than 16 tablets in 24 hours unless directed by a doctor
- do not use the maximum dose for more than 2 weeks
Children under 12 years of age: ask a doctor
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NUTRALOX
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0151 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MINERAL OIL (UNII: T5L8T28FGP) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;036 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0151-3 20 in 1 BOX, UNIT-DOSE 01/20/1987 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:50332-0151-4 50 in 1 BOX, UNIT-DOSE 01/20/1987 2 2 in 1 PACKET; Type 0: Not a Combination Product 3 NDC:50332-0151-7 125 in 1 BOX, UNIT-DOSE 01/20/1987 3 2 in 1 PACKET; Type 0: Not a Combination Product 4 NDC:50332-0151-8 250 in 1 BOX, UNIT-DOSE 01/20/1987 4 2 in 1 PACKET; Type 0: Not a Combination Product 5 NDC:50332-0151-1 500 in 1 BOX, UNIT-DOSE 01/20/1987 5 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 01/20/1987 Labeler - HART Health (069560969)