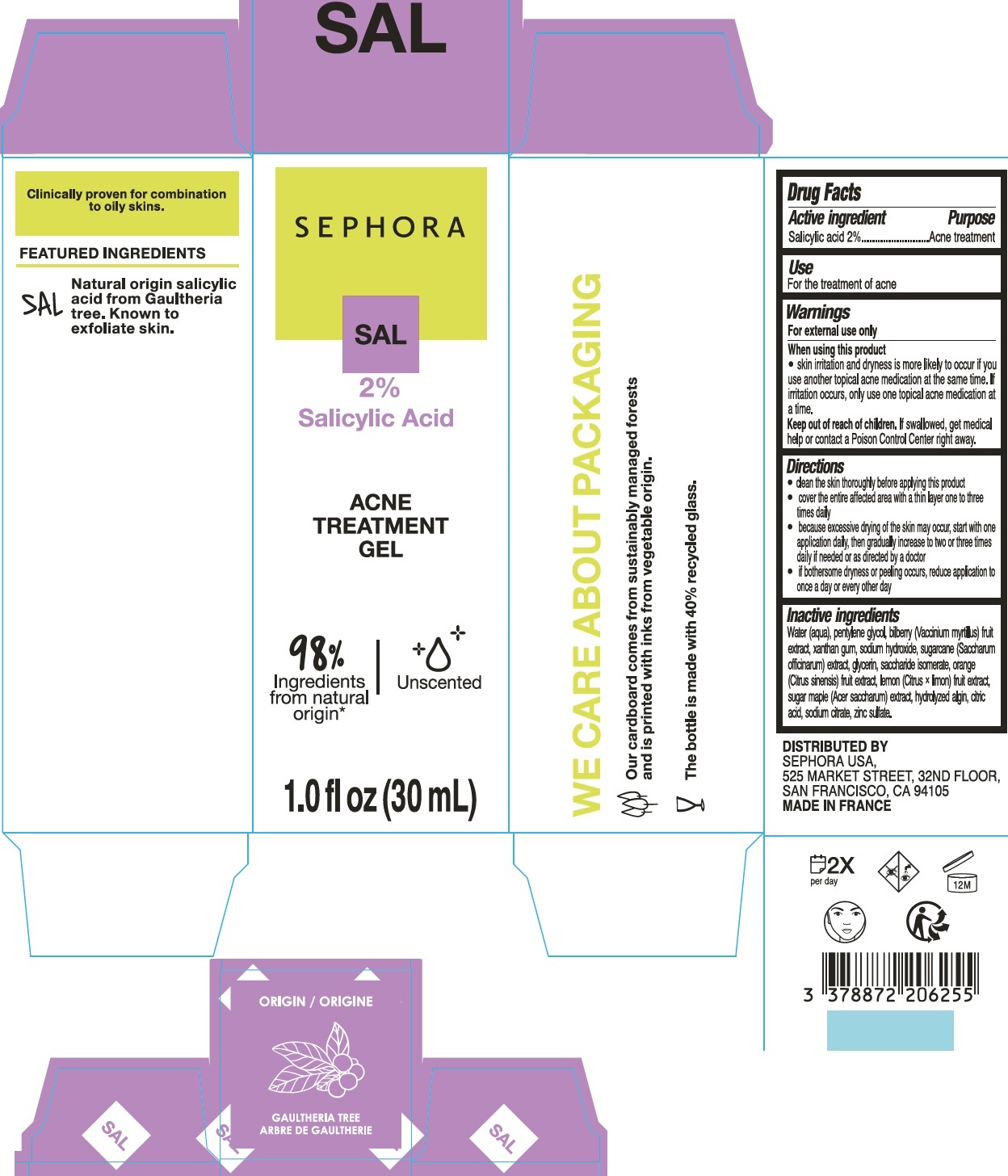
Label: ACNE TREATMENT GEL- salicylic acid gel
- NDC Code(s): 31720-301-08
- Packager: S+
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 31, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
Inactive ingredients
Water (aqua), pentylene glycol, biberry (Vaccinium myrtilus) fruit extract, xanthan gum, sodium hydroxide, sugarcane (Saccharum officinarum) extract, glycerin, saccharide isomerate, orange (Citrus sinensis) fruit extract, lemon (Citrus x limon) fruit extract, sugar maple (Acer saccharum) extract, hydrolyzed algin, citric acid, sodium citrate, zinc sulfate.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ACNE TREATMENT GEL
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:31720-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PENTYLENE GLYCOL (UNII: 50C1307PZG) MYRICA CERIFERA FRUIT (UNII: 2VZ27D14UH) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) SUGARCANE (UNII: 81H2R5AOH3) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIDE ISOMERATE (UNII: W8K377W98I) ORANGE (UNII: 5EVU04N5QU) LEMON (UNII: 24RS0A988O) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) ZINC SULFATE (UNII: 89DS0H96TB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31720-301-08 1 in 1 BOX 12/05/2022 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/05/2022 Labeler - S+ (572406531)