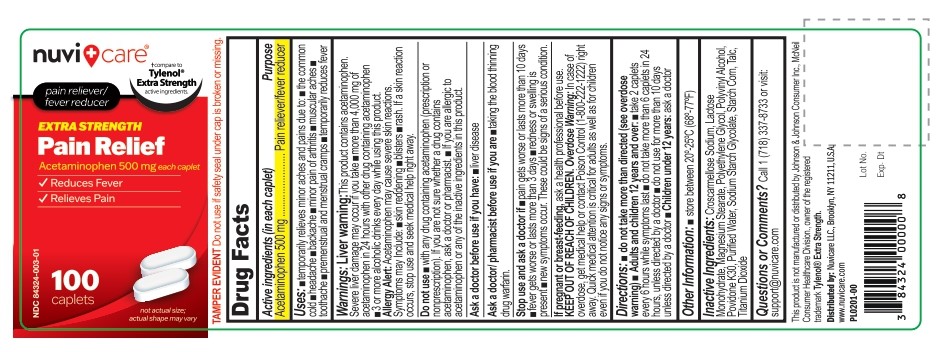
Label: ACETAMINOPHEN 500 MG- acetaminophen tablet
- NDC Code(s): 84324-003-01
- Packager: NUVICARE LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each caplet)
- Purpose
- Uses
- Warnings
- OTHER SAFETY INFORMATION
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- do not take more than directed (see overdose warning)
Adults and children 12 years and over:
- take 2 Caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
Children under 12 Years
- ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN 500 MG
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84324-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K30 (UNII: U725QWY32X) WATER (UNII: 059QF0KO0R) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape capsule Size 17mm Flavor Imprint Code P500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84324-003-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 07/17/2024 Labeler - NUVICARE LLC (119257565) Registrant - NUVICARE LLC (119257565) Establishment Name Address ID/FEI Business Operations TRUECARE BIOMEDIX 650526341 manufacture(84324-003) Establishment Name Address ID/FEI Business Operations MAK Pharma USA 109960731 pack(84324-003)