Label: BRODA ACNE- salicylic acid gel
- NDC Code(s): 54111-112-50, 54111-112-51
- Packager: Bentley Laboratories, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 27, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active Ingredient
- Purpose
- Keep out of reach of children
- Uses
- Warnings
- When using this product
-
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Inactive ingredients
-
BRODA Acne Spot Treatment and Acne Treatment Pen Labels
Tough on acne
Gentle on your skin
Anti-acne IntelliGel™
BRODA Acne Spot TreatmentBroda Acne Treatment Pen
IntelliGel™ Technology
Suitable for sensitive skinMaximum strength with 2% beta hydrox acid
Forms TrueSkin invisible patch
No peeling or flakingWon't dry your skin
Alcohol-free Hydrogel
NET WT. 0.071 oz. (2g) - 0.71 oz. (20 g)
Distributed by:
Broda International, LLC
10 Barley Court
Plainsboro, NJ 08536
Manufactured and packaged in the USA
www.broda.com
Patent pending
BRODA™
Anti-acne IntelliGel™
Developed with a proprietary break-through IntellGel™ technology that delivers maximum strength acne medication to effectively penetrate pores to clear most acne blemishes and help prevent new acne pimples.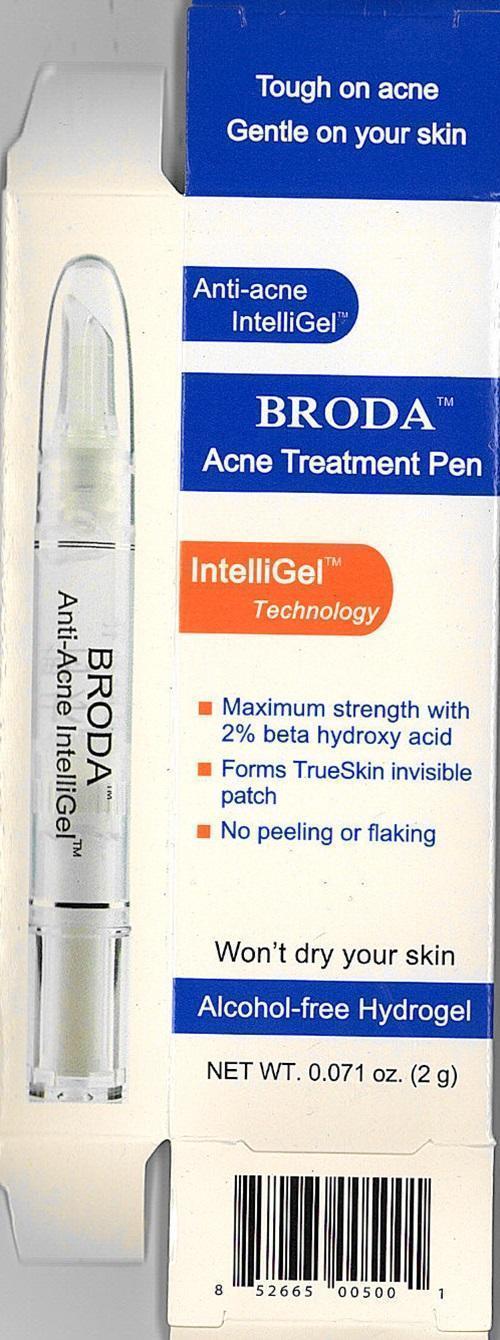


res
-
INGREDIENTS AND APPEARANCE
BRODA ACNE
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54111-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.4 g in 20 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) LAURETH-4 (UNII: 6HQ855798J) MENTHOL (UNII: L7T10EIP3A) LEVOMENOL (UNII: 24WE03BX2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54111-112-50 20 g in 1 TUBE; Type 0: Not a Combination Product 10/29/2013 2 NDC:54111-112-51 2 g in 1 TUBE; Type 0: Not a Combination Product 10/29/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 10/29/2013 Labeler - Bentley Laboratories, LLC (068351753) Establishment Name Address ID/FEI Business Operations Bentley Laboratories, LLC 068351753 manufacture(54111-112)



