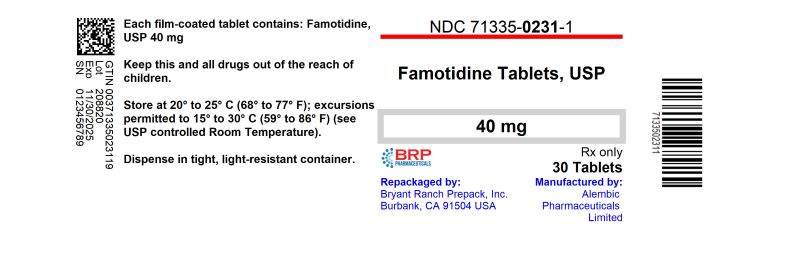
Label: FAMOTIDINE tablet
- NDC Code(s): 71335-0231-1, 71335-0231-2, 71335-0231-3, 71335-0231-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 62332-002
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 30, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FAMOTIDINE TABLETS safely and effectively. See full prescribing information for FAMOTIDINE TABLETS. FAMOTIDINE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFamotidine tablets are indicated in adult and pediatric patients 40 kg and greater for the treatment of: active duodenal ulcer (DU). active gastric ulcer (GU). symptomatic nonerosive ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Table 1 shows the recommended dosage of famotidine 20 mg and 40 mg tablets in adult and pediatric patients weighing 40 kg and greater with normal renal function. The use ...
-
3 DOSAGE FORMS AND STRENGTHS20 mg tablets: Yellow colored, circular, biconvex film-coated tablets embossed with ‘L113’on one side and ‘20’ on the other side. 40 mg tablets: Brown colored, circular, biconvex film-coated ...
-
4 CONTRAINDICATIONS(What is this?)Famotidine tablets are contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis) to famotidine or other histamine-2 (H2) receptor antagonists.
-
5 WARNINGS AND PRECAUTIONS5.1 Central Nervous System Adverse Reactions - Central nervous system (CNS) adverse reactions, including confusion, delirium, hallucinations, disorientation, agitation, seizures, and lethargy ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONS7.1 Drugs Dependent on Gastric pH for Absorption - Famotidine can reduce the absorption of other drugs, due to its effect on reducing intragastric acidity, leading to loss of efficacy of the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data with H2-receptor antagonists, including famotidine, in pregnant women are insufficient to establish a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEThe types of adverse reactions in overdosage of famotidine tablets are similar to the adverse reactions encountered with use of recommended dosages [see Adverse Reactions (6.1)]. In the event of ...
-
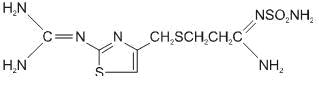
11 DESCRIPTIONThe active ingredient in famotidine tablets, USP is a histamine-2 (H2) receptor antagonist. Famotidine is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Famotidine is a competitive inhibitor of histamine-2 (H2) receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic potential of famotidine was assessed in a 106-week oral carcinogenicity study in rats and a 92-week oral carcinogenicity ...
-
14 CLINICAL STUDIES14.1 Active Duodenal Ulcer - In a U.S. multicenter, double-blind trial in adult outpatients with endoscopically confirmed duodenal ulcer (DU), orally administered famotidine tablets were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNDC: 71335-0231-1: 30 Tablets in a BOTTLE - NDC: 71335-0231-2: 60 Tablets in a BOTTLE - NDC: 71335-0231-3: 100 Tablets in a BOTTLE - NDC: 71335-0231-4: 90 Tablets in a BOTTLE - NDC: 71335-0231-5: 15 ...
-
17 PATIENT COUNSELING INFORMATIONCentral Nervous System (CNS) Adverse Reactions - Advise elderly patients and those with moderate and severe renal impairment of the risk of CNS adverse reactions, including confusion, delirium ...
-
PRINCIPAL DISPLAY PANEL(What is this?)Famotidine 40mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information