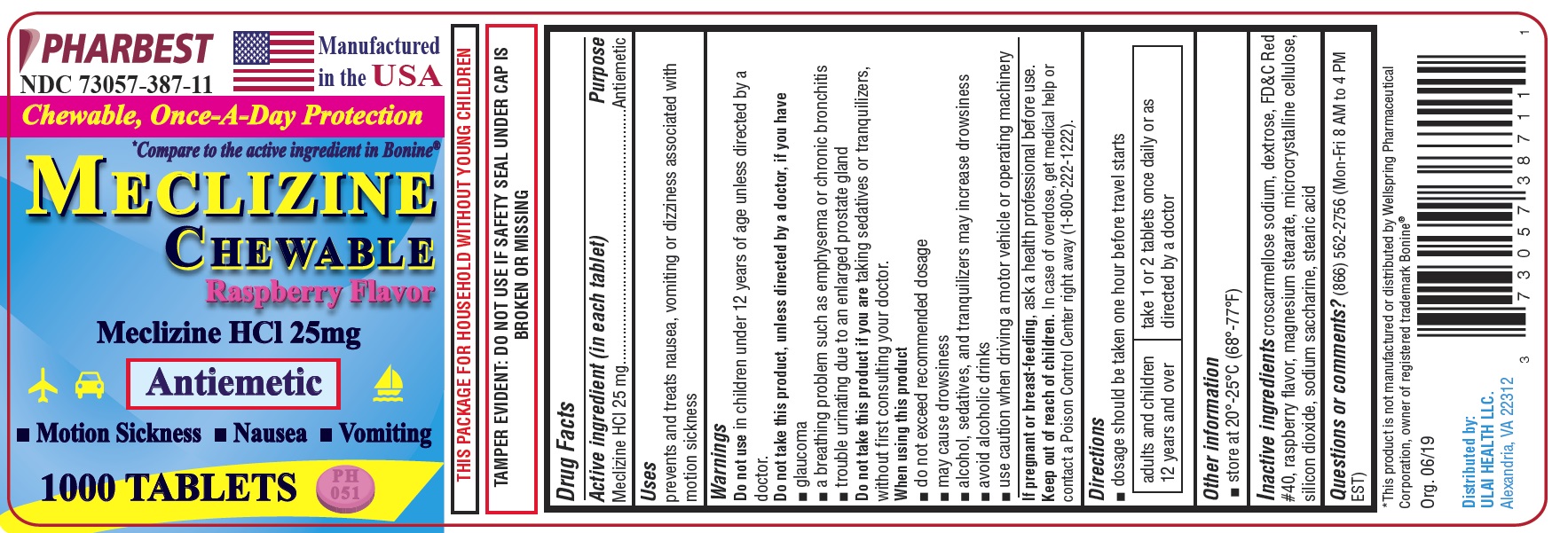
Label: MECLIZINE- meclizine hcl 25mg tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 73057-387-08, 73057-387-11 - Packager: Ulai Health LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 22, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
-
Do not use
in children under 12 years of age unless directed by a doctor.
Do not take this product, unless directed by a doctor, if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
- When using this product
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MECLIZINE
meclizine hcl 25mg tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73057-387 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SACCHARIN SODIUM (UNII: SB8ZUX40TY) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color pink (LIGHT PINK COLOR) Score 2 pieces Shape ROUND (Round Tablet) Size 8mm Flavor RASPBERRY Imprint Code PH051 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73057-387-11 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/08/2019 2 NDC:73057-387-08 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/08/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part336 07/08/2019 Labeler - Ulai Health LLC (081181535) Registrant - Pharbest Pharmaceuticals, Inc. (557054835) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 manufacture(73057-387) , analysis(73057-387) , pack(73057-387) , label(73057-387)