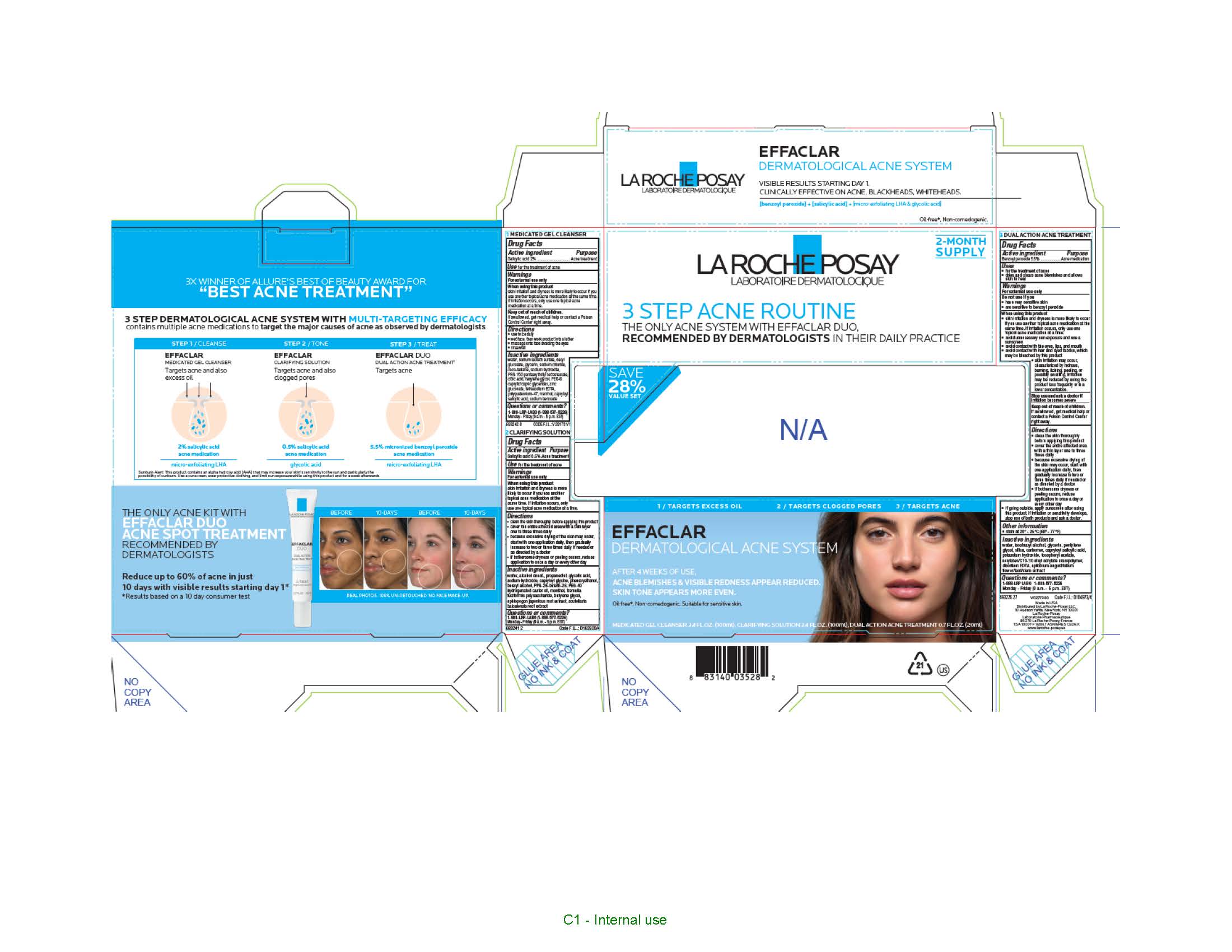
Label: LA ROCHE POSAY EFFACLAR DERMATOLOGICAL ACNE SYSTEM 3 STEP ACNE ROUTINE- benzoyl peroxide and salicylic acid kit
- NDC Code(s): 49967-282-01
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 21, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Effaclar Medicated Gel Cleanser
- Active ingredient
- Purpose
- Use
- Warnings
- When using this product
- Keep out of reach of children.
- Directions
-
Inactive Ingredients
water, sodium laureth sulfate, decyl glucoside, glycerin, sodium chloride, coco-betaine, sodium hydroxide, PEG-150 pentaerythrityl tetrastearate, citric acid, hexylene glycol, PEG-6 caprylic/capric glycerides, zinc gluconate, tetrasodium EDTA, polyquaternium-47, menthol, capryloyl salicylic acid, sodium benzoate
- Questions or comments?
- Effaclar Clarifying Solution
- Active ingredient
- Purpose
- Use
- Warnings
- When using this product
- Keep out of reach of children.
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
-
Inactive Ingredients
water, alcohol denat., propanediol, glycolic acid, sodium hydroxide, capryloyl glycine, phenoxyethanol, benzyl alcohol, PPG-26-buteth-26, PEG-40 hydrogenated castor oil, menthol, tremella fuciformis polysaccharide, butylene glycol, ophiopogon japonicus root extract, scutellaria baicalensis root extract
- Questions or comments?
- Effaclar Duo Dual Action Acne Treatment
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use if you
-
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- cleanse the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Other information
- Ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY EFFACLAR DERMATOLOGICAL ACNE SYSTEM 3 STEP ACNE ROUTINE
benzoyl peroxide and salicylic acid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-282 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-282-01 1 in 1 CARTON 01/01/2015 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 100 mL Part 2 1 BOTTLE 100 mL Part 3 1 TUBE 20 mL Part 1 of 3 LA ROCHE POSAY EFFACLAR MEDICATED GEL CLEANSER
salicylic acid gelProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) COCO-BETAINE (UNII: 03DH2IZ3FY) SODIUM HYDROXIDE (UNII: 55X04QC32I) PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PEG-6 CAPRYLIC/CAPRIC GLYCERIDES (UNII: GO50W2HWO8) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) EDETATE SODIUM (UNII: MP1J8420LU) POLYQUATERNIUM-47 (METHACRYLAMIDOPROPYLTRIMETHYLAMMONIUM CHLORIDE-CO-METHYL ACRYLATE-CO-ACRYLIC ACID 46:8:46; 1300000 MW) (UNII: G938900MID) MENTHOL (UNII: L7T10EIP3A) CAPRYLOYL SALICYLIC ACID (UNII: 5F7PJF6AA4) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 Part 2 of 3 LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE EFFACLAR CLARIFYING
salicylic acid solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) PROPANEDIOL (UNII: 5965N8W85T) GLYCOLIC ACID (UNII: 0WT12SX38S) SODIUM HYDROXIDE (UNII: 55X04QC32I) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZYL ALCOHOL (UNII: LKG8494WBH) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) MENTHOL (UNII: L7T10EIP3A) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 Part 3 of 3 LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE EFFACLAR DUO ACNE TREATMENT
benzoyl peroxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 55 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) GLYCERIN (UNII: PDC6A3C0OX) PENTYLENE GLYCOL (UNII: 50C1307PZG) SILICA (UNII: ETJ7Z6XBU4) CARBOMER (UNII: 0A5MM307FC) CAPRYLOYL SALICYLIC ACID (UNII: 5F7PJF6AA4) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S AT 0.5%) (UNII: YY2HMJ9NZF) EDETATE DISODIUM (UNII: 7FLD91C86K) EPILOBIUM ANGUSTIFOLIUM FLOWER/LEAF/STEM EXTRACT (UNII: 08H094218D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 20 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/01/2015 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 185931458 MANUFACTURE(49967-282) Establishment Name Address ID/FEI Business Operations Beauty Manufacturing Solutions Corp. 783200723 manufacture(49967-282)