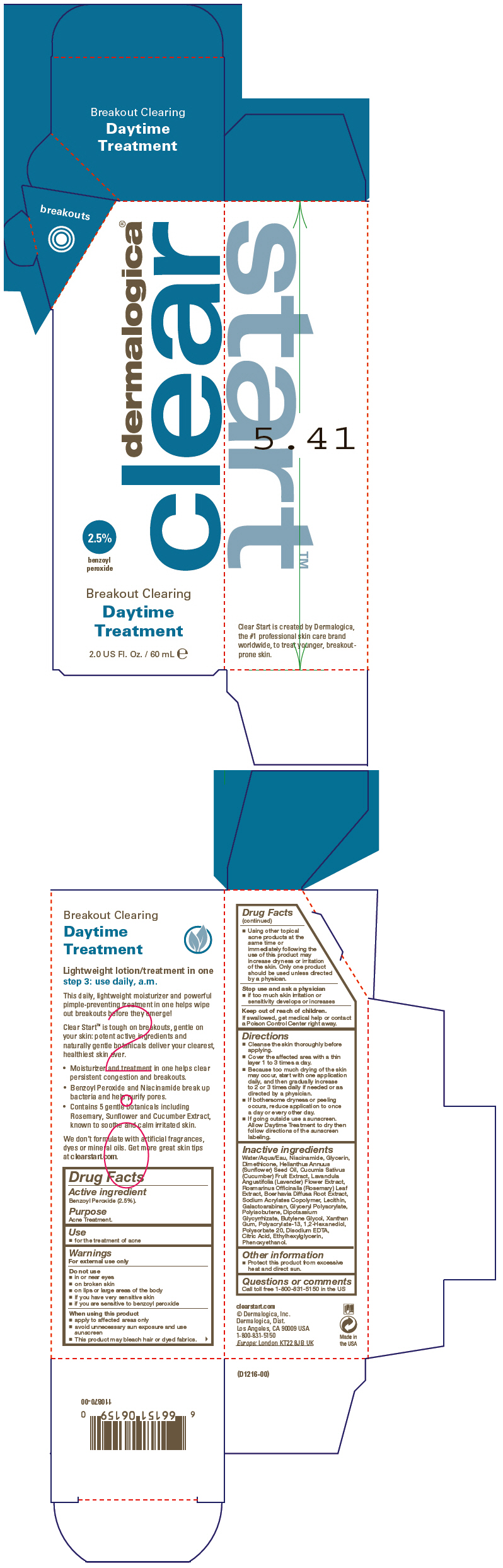
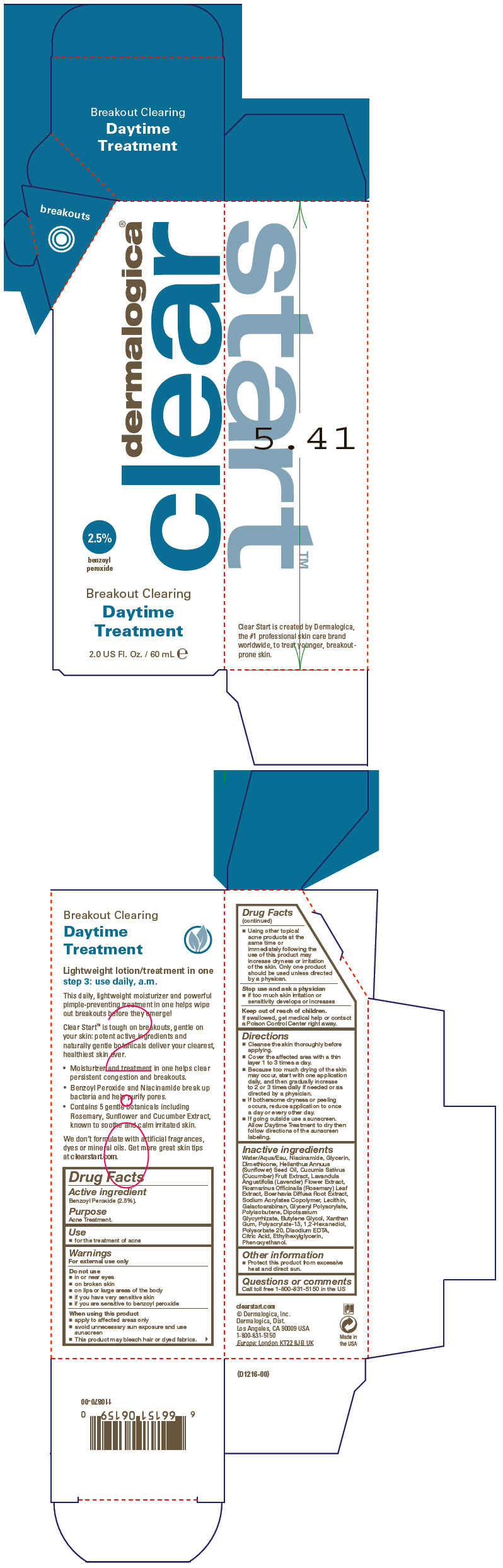
Label: BREAKOUT CLEARING DAYTIME TREATMENT- benzoyl peroxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 68479-160-00, 68479-160-01, 68479-160-02 - Packager: Dermalogica, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 24, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredientBenzoyl Peroxide (2.5%).
-
PurposeAcne Treatment.
-
Usefor the treatment of acne
-
WarningsFor external use only - Do not use - in or near eyes - on broken skin - on lips or large areas of the body - if you have very sensitive skin - if you are sensitive to benzoyl peroxide - When using ...
-
DirectionsCleanse the skin thoroughly before applying. Cover the affected area with a thin layer 1 to 3 times a day. Because too much drying of the skin may occur, start with one application daily, and ...
-
Inactive ingredientsWater/Aqua/Eau, Niacinamide, Glycerin, Dimethicone, Helianthus Annuus (Sunflower) Seed Oil, Cucumis Sativus (Cucumber) Fruit Extract, Lavandula Angustifolia (Lavender) Flower Extract, Rosmarinus ...
-
Other informationProtect this product from excessive heat and direct sun.
-
Questions or commentsCall toll free 1-800-831-5150 in the US
-
PRINCIPAL DISPLAY PANEL - 60 mL Tube Cartondermalogica® clear - 2.5% benzoyl - peroxide - Breakout Clearing - Daytime - Treatment - 2.0 US Fl. Oz. / 60 mL e
-
INGREDIENTS AND APPEARANCEProduct Information