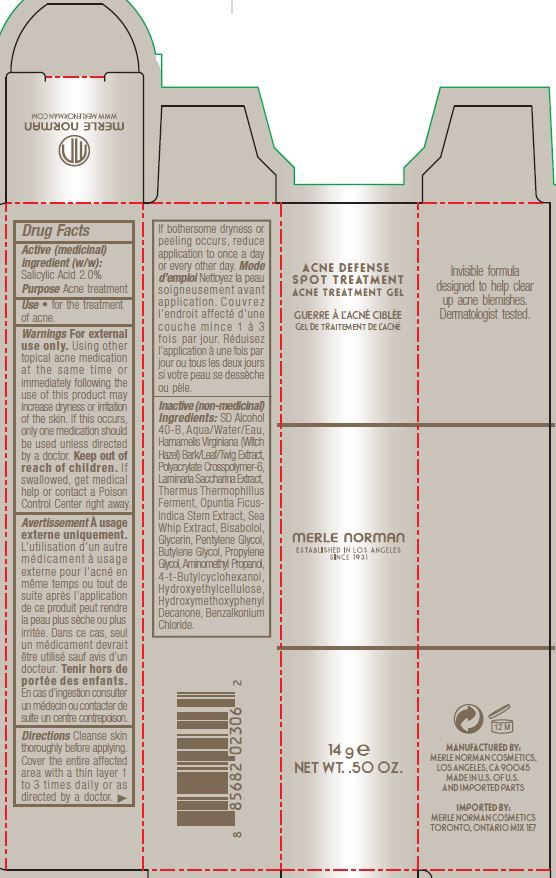
Label: ACNE DEFENSE SPOT TREATMENT MERLE NORMAN- salicylic acid gel
- NDC Code(s): 57627-201-01, 57627-201-02
- Packager: Merle Norman Cosmetics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
SD Alcohol 40-B, Aqua/Water/Eau, Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, Polyacrylate Crosspolymer 6, Laminaria Saccarina Extract, Thermus Thermophillus Ferment, Opuntia Ficus-Indica Stem Extract, Sea Whip Extract, Bisabolol, Glycerin, Pentylene Glycol, Butylene Glycol, Propylene Glycol, Aminomethyl Propanol, 4-t-Butylcyclohexanol, Hydroxyethylcellulose, Hydroxymethoxyphenyl Decanone, Benzalkonium Chloride
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE DEFENSE SPOT TREATMENT MERLE NORMAN
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57627-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) OPUNTIA FICUS-INDICA STEM (UNII: MUD8892KHL) PSEUDOPTEROGORGIA ELISABETHAE (UNII: UDY3H1OUX5) LEVOMENOL (UNII: 24WE03BX2T) GLYCERIN (UNII: PDC6A3C0OX) PENTYLENE GLYCOL (UNII: 50C1307PZG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) 4-TERT-BUTYLCYCLOHEXANOL (UNII: K0H1405S9C) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) 1-(4-HYDROXY-3-METHOXYPHENYL)-DECAN-3-ONE (UNII: BO24ID7E9U) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57627-201-02 1 in 1 CARTON 07/14/2017 1 NDC:57627-201-01 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/14/2017 Labeler - Merle Norman Cosmetics, Inc (008479388)