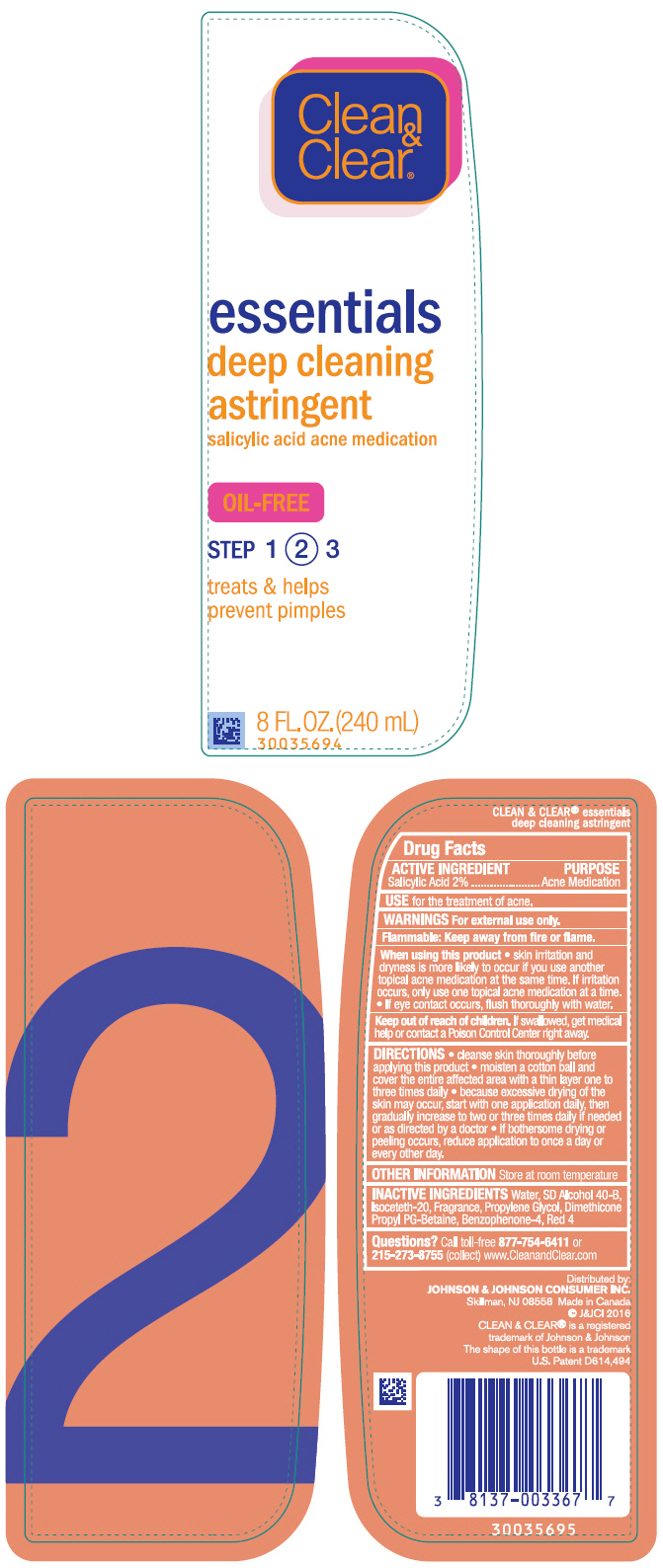
Label: CLEAN AND CLEAR ESSENTIALS DEEP CLEANING ASTRINGENT- salicylic acid liquid
- NDC Code(s): 69968-0209-8
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- USE
-
WARNINGS
For external use only.
Flammable: Keep away from fire or flame.
-
DIRECTIONS
- cleanse skin thoroughly before applying this product
- moisten a cotton ball and cover the entire effected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome drying or peeling occurs, reduce application to once a day or every other day.
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 240 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
CLEAN AND CLEAR ESSENTIALS DEEP CLEANING ASTRINGENT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0209 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE PROPYL PG-BETAINE (UNII: OB83A4S9K9) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) ISOCETETH-20 (UNII: O020065R7Z) SULISOBENZONE (UNII: 1W6L629B4K) FD&C RED NO. 4 (UNII: X3W0AM1JLX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0209-8 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/30/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 04/30/2012 Labeler - Kenvue Brands LLC (118772437)