Label: CAREONE ACID REDUCER COMPLETE- famotidine, calcium carbonate, magnesium hydroxide tablet, chewable
- NDC Code(s): 41520-617-63
- Packager: American Sales Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each chewable tablet)
- Purposes
- Use
-
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- •
- with other acid reducers
Ask a doctor before use if you have
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating, or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
- •
- kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Antacids and acid reducers may interact with certain prescription drugs.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
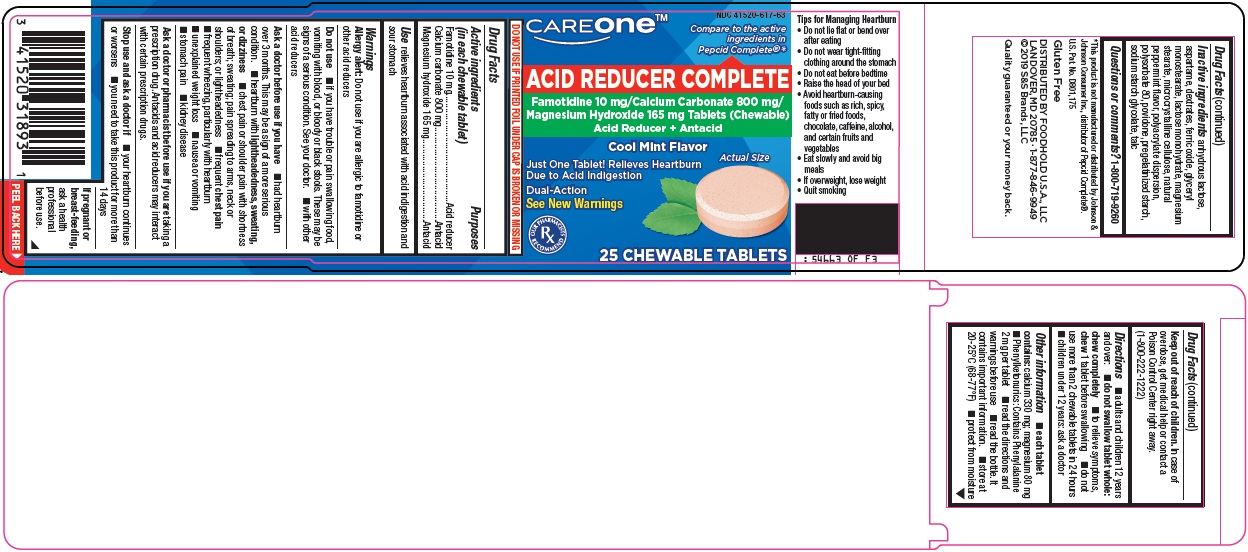
Principal Display Panel
CAREONE™
Compare to the active ingredients in Pepcid Complete®
ACID REDUCER COMPLETE
Famotidine 10 mg/Calcium Carbonate 800 mg/Magnesium Hydroxide 165 mg Tablets (Chewable)
Acid Reducer + Antacid
Cool Mint Flavor
Actual Size
Just One Tablet! Relieves Heartburn Due to Acid Indigestion
Dual-Action
See New Warnings
OUR PHARMACISTS RECOMMEND
25 CHEWABLE TABLETS
-
INGREDIENTS AND APPEARANCE
CAREONE ACID REDUCER COMPLETE
famotidine, calcium carbonate, magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41520-617 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 800 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 165 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) ASPARTAME (UNII: Z0H242BBR1) DEXTRATES (UNII: G263MI44RU) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color ORANGE (PEACH WITH WHITE SPECKLES) Score no score Shape ROUND Size 18mm Flavor MINT Imprint Code L546 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41520-617-63 25 in 1 BOTTLE; Type 0: Not a Combination Product 10/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077355 10/04/2016 Labeler - American Sales Company (809183973)