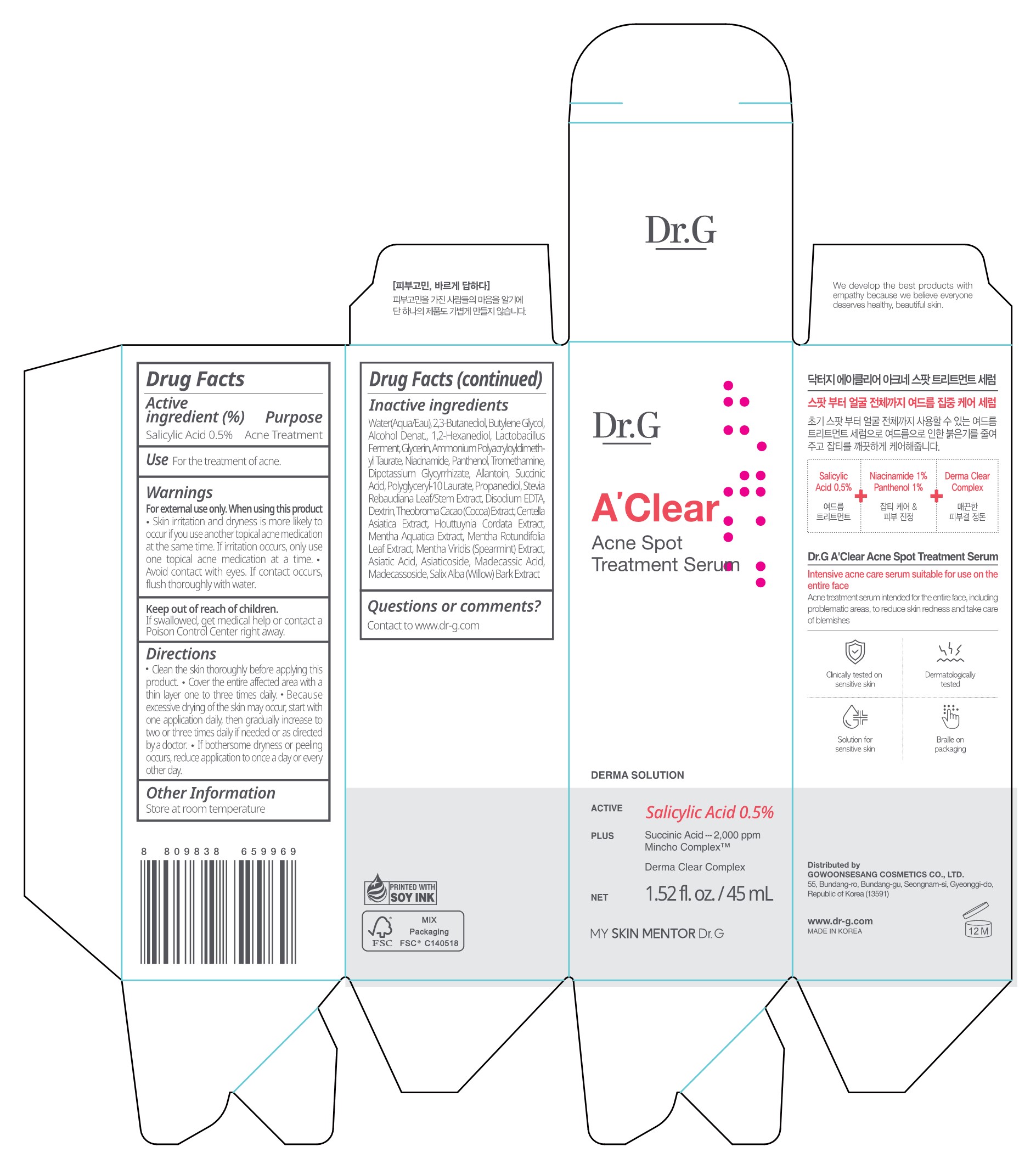
Label: DR.G A CLEAR ACNE SPOT TREATMENT SERUM- salicylic acid liquid
- NDC Code(s): 51621-412-01
- Packager: GOWOONSESANG COSMETICS CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only. When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time .
- Avoid contact with eyes. If contact occurs, flush thoroughly with water.
- Keep out of reach of children.
-
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other Information
-
Inactive ingredients
Water(Aqua/Eau), 2,3-Butanediol, Butylene Glycol, Alcohol Denat., 1,2-Hexanediol, Lactobacillus Ferment, Glycerin, Ammonium Polyacryloyldimeth yl Taurate, Niacinamide, Panthenol, Tromethamine, Dipotassium Glycyrrhizate, Allantoin, Succinic Acid, Polyglyceryl-10 Laurate, Propanediol, Stevia Rebaudiana Leaf/Stem Extract, Disodium EDTA, Dextrin, Theobroma Cacao (Cocoa) Extract, Centella Asiatica Extract, Houttuynia Cordata Extract, Mentha Aquatica Extract, Mentha Rotundifolia Leaf Extract, Mentha Viridis (Spearmint) Extract, Asiatic Acid, Asiaticoside, Madecassic Acid, Madecassoside, Salix Alba (Willow) Bark Extract
- Questions or comments?
- PACKAGE LABEL Dr.G A CLEAR ACNE SPOT TREATMENT SERUM 45ml
-
INGREDIENTS AND APPEARANCE
DR.G A CLEAR ACNE SPOT TREATMENT SERUM
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51621-412 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SUCCINIC ACID (UNII: AB6MNQ6J6L) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) COCOA (UNII: D9108TZ9KG) ALLANTOIN (UNII: 344S277G0Z) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) ALCOHOL (UNII: 3K9958V90M) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) MADECASSOSIDE (UNII: CQ2F5O6YIY) DEXTRIN, CORN (UNII: VAD2K35XAJ) PANTHENOL (UNII: WV9CM0O67Z) PROPANEDIOL (UNII: 5965N8W85T) AMMONIUM POLYACRYLOYLDIMETHYL TAURATE (UNII: F01RIY4371) NIACINAMIDE (UNII: 25X51I8RD4) ASIATIC ACID (UNII: 9PA5A687X5) TROMETHAMINE (UNII: 023C2WHX2V) SALIX ALBA BARK (UNII: 205MXS71H7) MENTHA X ROTUNDIFOLIA LEAF (UNII: K59TXG2L3U) DIPOTASSIUM GLYCYRRHIZATE (UNII: CA2Y0FE3FX) MADECASSIC ACID (UNII: M7O1N24J82) WATER (UNII: 059QF0KO0R) 2,3-BUTANEDIOL (UNII: 45427ZB5IJ) EDETATE DISODIUM (UNII: 7FLD91C86K) MENTHA SPICATA (UNII: O2H83I4PUN) ASIATICOSIDE (UNII: PKO39VY215) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51621-412-01 1 in 1 BOX 12/01/2024 1 45 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2024 Labeler - GOWOONSESANG COSMETICS CO., LTD. (631170768)