Label: DOXORUBICIN HYDROCHLORIDE injection, solution
-
NDC Code(s):
0069-0277-02,
0069-0343-02,
0069-0358-20,
0069-1542-20, view more0069-3030-20, 0069-3031-20, 0069-3032-20, 0069-3033-20, 0069-3034-20
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DOXORUBICIN HYDROCHLORIDE safely and effectively. See full prescribing information for DOXORUBICIN HYDROCHLORIDE.
DOXORUBICIN HYDROCHLORIDE injection, for intravenous use
Initial U.S. Approval: 1974WARNING: CARDIOMYOPATHY, SECONDARY MALIGNANCIES, EXTRAVASATION AND TISSUE NECROSIS, and SEVERE MYELOSUPPRESSION
See full prescribing information for complete boxed warning.
- •
- Cardiomyopathy: Myocardial damage can occur with doxorubicin hydrochloride with incidences from 1%–20% for cumulative doses from 300 mg/m2 to 500 mg/m2 when doxorubicin hydrochloride is administered every 3 weeks. The risk of cardiomyopathy is further increased with concomitant cardiotoxic therapy. Assess left ventricular ejection fraction (LVEF) before and regularly during and after treatment with doxorubicin hydrochloride. (5.1)
- •
- Secondary Malignancies: Secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) occur at a higher incidence in patients treated with anthracyclines, including doxorubicin hydrochloride. (5.2)
- •
- Extravasation and Tissue Necrosis: Extravasation of doxorubicin hydrochloride can result in severe local tissue injury and necrosis requiring wide excision and skin grafting. Immediately terminate the drug, and apply ice to the affected area. (5.3)
- •
- Severe myelosuppression resulting in serious infection, septic shock, requirement for transfusions, hospitalization, and death may occur. (5.4)
INDICATIONS AND USAGE
Doxorubicin Hydrochloride Injection is an anthracycline topoisomerase inhibitor indicated:
- •
- as a component of multi‑agent adjuvant chemotherapy for treatment of women with axillary lymph node involvement following resection of primary breast cancer (1.1)
- •
- for the treatment of: acute lymphoblastic leukemia, acute myeloblastic leukemia, Hodgkin lymphoma, Non-Hodgkin lymphoma, metastatic breast cancer, metastatic Wilms' tumor, metastatic neuroblastoma, metastatic soft tissue sarcoma, metastatic bone sarcomas, metastatic ovarian carcinoma, metastatic transitional cell bladder carcinoma, metastatic thyroid carcinoma, metastatic gastric carcinoma, metastatic bronchogenic carcinoma (1.2)
DOSAGE AND ADMINISTRATION
- •
- Single agent: 60 to 75 mg/m2 given intravenously every 21 days (2.2)
- •
- In combination: 40 to 75 mg/m2 given intravenously every 21 to 28 days (2.2)
- •
- Discontinue Doxorubicin Hydrochloride Injection in patients who develop signs or symptoms of cardiomyopathy (2.3)
- •
- Reduce dose in patients with hepatic impairment (2.4)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Radiation-Induced Toxicity: Can be increased by the administration of Doxorubicin Hydrochloride Injection. Radiation recall can occur in patients who receive Doxorubicin Hydrochloride Injection after prior radiation therapy. (5.7)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and on the use of effective contraception. Advise males with female partners of reproductive potential to use effective contraception. Advise males with pregnant partners to use condoms. (5.8, 8.1, 8.3)
ADVERSE REACTIONS
The most common (>10%) adverse reactions are alopecia, nausea and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOMYOPATHY, SECONDARY MALIGNANCIES, EXTRAVASATION AND TISSUE NECROSIS, and SEVERE MYELOSUPPRESSION
1 INDICATIONS AND USAGE
1.1 Adjuvant Breast Cancer
1.2 Other Cancers
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adjuvant Breast Cancer
2.2 Recommended Dosage for Other Cancers
2.3 Dosage Modifications for Adverse Reactions
2.4 Dosage Modifications for Hepatic Impairment
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy and Arrhythmias
5.2 Secondary Malignancies
5.3 Extravasation and Tissue Necrosis
5.4 Severe Myelosuppression
5.5 Use in Patients with Hepatic Impairment
5.6 Tumor Lysis Syndrome
5.7 Potentiation of Radiation Toxicity and Radiation Recall
5.8 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Doxorubicin Hydrochloride Injection
7.2 Concomitant Use of Trastuzumab
7.3 Concomitant Use of Dexrazoxane
7.4 Concomitant Use of 6-Mercaptopurine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adjuvant Breast Cancer
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CARDIOMYOPATHY, SECONDARY MALIGNANCIES, EXTRAVASATION AND TISSUE NECROSIS, and SEVERE MYELOSUPPRESSION
- •
- Cardiomyopathy: Myocardial damage, including acute left ventricular failure, can occur with doxorubicin hydrochloride. The risk of cardiomyopathy is proportional to the cumulative exposure with incidence rates from 1%–20% for cumulative doses ranging from 300 mg/m2 to 500 mg/m2 when doxorubicin hydrochloride is administered every 3 weeks. The risk of cardiomyopathy is further increased with concomitant cardiotoxic therapy. Assess left ventricular ejection fraction (LVEF) before and regularly during and after treatment with doxorubicin hydrochloride [see Warnings and Precautions (5.1)].
- •
- Secondary Malignancies: Secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) occur at a higher incidence in patients treated with anthracyclines, including doxorubicin hydrochloride [see Warnings and Precautions (5.2)].
- •
- Extravasation and Tissue Necrosis: Extravasation of doxorubicin hydrochloride can result in severe local tissue injury and necrosis requiring wide excision of the affected area and skin grafting. Immediately terminate the drug and apply ice to the affected area [see Warnings and Precautions (5.3)].
- •
- Severe myelosuppression resulting in serious infection, septic shock, requirement for transfusions, hospitalization, and death may occur [see Warnings and Precautions (5.4)].
-
1 INDICATIONS AND USAGE
1.1 Adjuvant Breast Cancer
Doxorubicin Hydrochloride Injection is indicated as a component of multi-agent adjuvant chemotherapy for treatment of women with axillary lymph node involvement following resection of primary breast cancer.
1.2 Other Cancers
Doxorubicin Hydrochloride Injection is indicated for the treatment of
- •
- acute lymphoblastic leukemia
- •
- acute myeloblastic leukemia
- •
- Hodgkin lymphoma
- •
- non-Hodgkin lymphoma (NHL)
- •
- metastatic breast cancer
- •
- metastatic Wilms' tumor
- •
- metastatic neuroblastoma
- •
- metastatic soft tissue sarcoma
- •
- metastatic bone sarcoma
- •
- metastatic ovarian carcinoma
- •
- metastatic transitional cell bladder carcinoma
- •
- metastatic thyroid carcinoma
- •
- metastatic gastric carcinoma
- •
- metastatic bronchogenic carcinoma
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adjuvant Breast Cancer
The recommended dosage of Doxorubicin Hydrochloride Injection is 60 mg/m2 administered as an intravenous bolus on day 1 of each 21-day treatment cycle, in combination with cyclophosphamide, for a total of four cycles.
2.2 Recommended Dosage for Other Cancers
- •
- The recommended dosage of Doxorubicin Hydrochloride Injection when used as a single agent is 60 mg/m2 to 75 mg/m2 intravenously every 21 days.
- •
- The recommended dosage of Doxorubicin Hydrochloride Injection, when administered in combination with other chemotherapy drugs, is 40 mg/m2 to 75 mg/m2 intravenously every 21 to 28 days.
- •
- Consider use of the lower Doxorubicin Hydrochloride Injection dose in the recommended dosage range or longer intervals between cycles for heavily pretreated patients, elderly patients, or obese patients.
- •
- Cumulative doses above 550 mg/m2 are associated with an increased risk of cardiomyopathy [see Warnings and Precautions (5.1)].
2.3 Dosage Modifications for Adverse Reactions
Cardiomyopathy
Discontinue Doxorubicin Hydrochloride Injection in patients who develop signs or symptoms of cardiomyopathy [see Warnings and Precautions (5.1)].
2.4 Dosage Modifications for Hepatic Impairment
Doxorubicin Hydrochloride Injection is contraindicated in patients with severe hepatic impairment (Child-Pugh Class C or serum bilirubin greater than 5 mg/dL) [see Contraindications (4)].
Dosage modifications for Doxorubicin Hydrochloride Injection in patients with elevated serum total bilirubin concentrations [see Warnings and Precautions (5.5), Use in Specific Populations (8.6)] are provided in Table 1.
Table 1. Recommended Dosage Modification for Elevated Serum Total Bilirubin Serum Total Bilirubin Concentration Dosage Modification 1.2–3 mg/dL
50%
3.1–5 mg/dL
75%
Greater than 5 mg/dL
Do not initiate Doxorubicin Hydrochloride Injection;
discontinue Doxorubicin Hydrochloride Injection2.5 Preparation and Administration
Doxorubicin Hydrochloride Injection is a hazardous drug. Follow applicable special handling and disposal procedures.1
Preparation
Dilution of Doxorubicin Hydrochloride Injection
- •
- Dilute Doxorubicin Hydrochloride Injection in 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.
- •
- Protect from light following preparation until completion of infusion.
- •
- Use within 1 hour. If not used within 1 hour, discard the diluted product.
Administration
- •
- Visually inspect for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if the solution is discolored, cloudy, or contains particulate matter.
Administration by Intravenous Injection
- •
- Administer diluted Doxorubicin Hydrochloride Injection as an intravenous injection through a central intravenous line or a secure and free-flowing peripheral venous line containing 0.9% Sodium Chloride Injection, USP, 0.45% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP.
- •
- Administer intravenously over 3 to 10 minutes. Decrease the rate of infusion if erythematous streaking along the vein proximal to the site of infusion or facial flushing occur.
Administration by Continuous Intravenous Infusion
- •
- Administer diluted Doxorubicin Hydrochloride Injection solution only through a central intravenous line. Decrease the rate of infusion if erythematous streaking along the vein proximal to the site of infusion or facial flushing occur.
- •
- Protect from light from preparation for infusion until completion of infusion.
Management of Suspected Extravasation
Immediately discontinue Doxorubicin Hydrochloride Injection for burning or stinging sensation or other evidence indicating peri-venous infiltration or extravasation. Manage confirmed or suspected extravasation as follows:
- •
- Do not remove the needle until attempts are made to aspirate extravasated fluid.
- •
- Do not flush the line.
- •
- Avoid applying pressure to the site.
- •
- Apply ice to the site intermittently for 15 minutes, 4 times a day for 3 days.
- •
- If the extravasation is in an extremity, elevate the extremity.
- •
- In adults, consider administration of dexrazoxane [see Warnings and Precautions (5.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Doxorubicin Hydrochloride Injection are contraindicated in patients with:
- •
- Severe myocardial insufficiency [see Warnings and Precautions (5.1)]
- •
- Recent (occurring within the past 4-6 weeks) myocardial infarction [see Warnings and Precautions (5.1)]
- •
- Severe persistent drug-induced myelosuppression [see Warnings and Precautions (5.4)]
- •
- Severe hepatic impairment (defined as Child Pugh Class C or serum bilirubin level greater than 5 mg/dL) [see Warnings and Precautions (5.5)]
- •
- Severe hypersensitivity reaction to doxorubicin hydrochloride, including anaphylaxis [see Adverse Reactions (6.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy and Arrhythmias
Cardiomyopathy
Doxorubicin hydrochloride can result in myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy is generally proportional to the cumulative exposure. Include prior doses of other anthracyclines or anthracenediones in calculations of total cumulative dosage for doxorubicin hydrochloride. Cardiomyopathy may develop during treatment or up to several years after completion of treatment and can include decrease in LVEF and signs and symptoms of congestive heart failure (CHF). The probability of developing cardiomyopathy is estimated to be 1 to 2% at a total cumulative dose of 300 mg/m2 of doxorubicin hydrochloride, 3 to 5% at a dose of 400 mg/m2, 5 to 8% at a dose of 450 mg/m2, and 6 to 20% at a dose of 500 mg/m2, when doxorubicin hydrochloride is administered every 3 weeks. There is an additive or potentially synergistic increase in the risk of cardiomyopathy in patients who have received radiotherapy to the mediastinum or concomitant therapy with other known cardiotoxic agents, such as cyclophosphamide and trastuzumab.
Pericarditis and myocarditis have also been reported during or following doxorubicin hydrochloride treatment.
Assess left ventricular cardiac function (e.g., MUGA or echocardiogram) prior to initiation of Doxorubicin Hydrochloride Injection, during treatment to detect acute changes, and after treatment to detect delayed cardiotoxicity. Increase the frequency of assessments as the cumulative dose exceeds 300 mg/m2. Use the same method of assessment of LVEF at all time points [see Use in Specific Populations (8.4)]. Discontinue Doxorubicin Hydrochloride Injection in patients who develop signs or symptoms of cardiomyopathy [see Dosage and Administration (2.3)].
Consider the use of dexrazoxane to reduce the incidence and severity of cardiomyopathy due to doxorubicin hydrochloride administration in patients who have received a cumulative doxorubicin hydrochloride dose of 300 mg/m2 and who will continue to receive doxorubicin hydrochloride.
Arrhythmias
Doxorubicin hydrochloride can result in arrhythmias, including life-threatening arrhythmias, during or within a few hours after doxorubicin hydrochloride administration and at any time point during treatment. Tachyarrhythmias, including sinus tachycardia, premature ventricular contractions, and ventricular tachycardia, as well as bradycardia, can occur. Electrocardiographic changes, including non-specific ST-T wave changes, atrioventricular and bundle-branch block can also occur. These electrocardiographic changes may be transient and self-limited and may not require a dosage modification of doxorubicin hydrochloride.
5.2 Secondary Malignancies
The risk of developing secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) is increased following treatment with doxorubicin hydrochloride. Cumulative incidences ranged from 0.2% at five years to 1.5% at 10 years in two separate trials involving the adjuvant treatment of women with breast cancer. These leukemias generally occur within 1 to 3 years of treatment.
5.3 Extravasation and Tissue Necrosis
Extravasation of doxorubicin hydrochloride can cause severe local tissue injury manifesting as blistering, ulceration, and necrosis requiring wide excision of the affected area and skin grafting. Extravasation should be considered if a patient experiences a burning or stinging sensation or shows other evidence indicating peri-venous infiltration or extravasation; however, extravasation may be present in patients who do not experience a stinging or burning sensation or when blood return is present on aspiration of the infusion needle.
When given via a peripheral venous line, infuse Doxorubicin Hydrochloride Injection over 10 minutes or less to minimize the risk of thrombosis or perivenous extravasation.
If extravasation is suspected, immediately discontinue the intravenous injection or continuous intravenous infusion [see Dosage and Administration (2.5)]. Apply ice to the site intermittently for 15 minutes, 4 times a day for 3 days. In adults, if appropriate, administer dexrazoxane at the site of extravasation as soon as possible and within the first 6 hours after extravasation.
5.4 Severe Myelosuppression
Doxorubicin hydrochloride can cause myelosuppression. In Study 1, the incidence of severe myelosuppression was: grade 4 leukopenia (0.3%), grade 3 leukopenia (3%), and grade 4 thrombocytopenia (0.1%). A dose-dependent, reversible neutropenia is the predominant manifestation of myelosuppression from doxorubicin hydrochloride. When doxorubicin hydrochloride is administered every 21 days, the neutrophil count reaches its nadir 10 to 14 days after administration with recovery usually occurring by day 21.
Obtain complete blood counts prior to each treatment and carefully monitor patients during treatment for possible clinical complications due to myelosuppression. Delay next dose of Doxorubicin Hydrochloride Injection if severe myelosuppression has not improved. Consider dose reduction for patients with prolonged myelosuppression based on severity of reaction.
5.5 Use in Patients with Hepatic Impairment
The clearance of doxorubicin is decreased in patients with elevated serum bilirubin with an increased risk of toxicity [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. Doxorubicin Hydrochloride Injection is contraindicated in patients with severe hepatic impairment (defined as Child Pugh Class C or serum bilirubin level greater than 5 mg/dL) [see Contraindications (4)]. Reduce the dose of Doxorubicin Hydrochloride Injection in patients with serum bilirubin levels of 1.2 to 5 mg/dL [see Dosage and Administration (2.4)]. Obtain liver tests including ALT, AST, alkaline phosphatase, and bilirubin prior to and during therapy.
5.6 Tumor Lysis Syndrome
Doxorubicin hydrochloride can induce tumor lysis syndrome in patients with rapidly growing tumors. Evaluate blood uric acid levels, potassium, calcium, phosphate, and creatinine after initial treatment. Hydration, urine alkalinization, and prophylaxis with allopurinol to prevent hyperuricemia may minimize potential complications of tumor lysis syndrome.
5.7 Potentiation of Radiation Toxicity and Radiation Recall
Doxorubicin hydrochloride can increase radiation-induced toxicity to the myocardium, mucosa, skin, and liver. Radiation recall, including but not limited to cutaneous and pulmonary toxicity, can occur in patients who receive doxorubicin hydrochloride after prior radiation therapy.
5.8 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, Doxorubicin Hydrochloride Injection can cause fetal harm when administered to a pregnant woman; avoid the use of Doxorubicin Hydrochloride Injection during the 1st trimester. Available human data do not establish the presence or absence of major birth defects and miscarriage related to the use of doxorubicin hydrochloride during the 2nd and 3rd trimesters. Doxorubicin hydrochloride was teratogenic and embryotoxic in rats and rabbits at doses lower than the recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Doxorubicin Hydrochloride Injection and for 6 months after treatment. Advise males with female partners of reproductive potential to use effective contraception during treatment with Doxorubicin Hydrochloride Injection and for 3 months after treatment. Advise males with pregnant partners to use condoms during treatment with Doxorubicin Hydrochloride Injection and for at least 10 days after the final dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- •
- Cardiomyopathy and Arrhythmias [see Warnings and Precautions (5.1)]
- •
- Secondary Malignancies [see Warnings and Precautions (5.2)]
- •
- Extravasation and Tissue Necrosis [see Warnings and Precautions (5.3)]
- •
- Severe Myelosuppression [see Warnings and Precautions (5.4)]
- •
- Tumor Lysis Syndrome [see Warnings and Precautions (5.6)]
- •
- Radiation Sensitization and Radiation Recall [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Breast Cancer
The safety data below were collected from 1492 women who received doxorubicin hydrochloride at a dose of 60 mg/m2 and cyclophosphamide at a dose of 600 mg/m2 (AC) every 3 weeks for 4 cycles for the adjuvant treatment of axillary lymph node positive breast cancer. The median number of cycles received was 4. Selected adverse reactions reported in this study are provided in Table 2. No treatment-related deaths were reported in patients on either arm of the study.
Table 2. Selected Adverse Reactions in Patients with Early Breast Cancer Involving Axillary Lymph Nodes Adverse Reactions AC*
N = 1492Conventional CMF
N = 739% % AC = doxorubicin hydrochloride, cyclophosphamide; CMF = cyclophosphamide, methotrexate, fluorouracil - *
- Includes pooled data from patients who received either AC for 4 cycles or AC for 4 cycles followed by CMF for 3 cycles
Alopecia
92
71
Vomiting
Vomiting ≤12 hours
34
25
Vomiting >12 hours
37
12
Intractable
5
2
Leukopenia
Grade 3 (1,000–1,999 /mm3)
3.4
9.4
Grade 4 (<1000 /mm3)
0.3
0.3
Shock, sepsis
2
1
Systemic infection
2
1
Cardiac dysfunction
Asymptomatic
0.2
0.1
Transient
0.1
0
Symptomatic
0.1
0
Thrombocytopenia
Grade 3 (25,000–49,999 /mm3)
0
0.3
Grade 4 (<25,000 /mm3)
0.1
0
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Doxorubicin Hydrochloride Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac – Cardiogenic shock
Cutaneous – Skin and nail hyperpigmentation, oncolysis, rash, itching, photosensitivity, urticaria, acral erythema, palmar plantar erythrodysesthesia
Gastrointestinal – Nausea, mucositis, stomatitis, necrotizing colitis, typhlitis, gastric erosions, gastrointestinal tract bleeding, hematochezia, esophagitis, anorexia, abdominal pain, dehydration, diarrhea, hyperpigmentation of the oral mucosa
Hypersensitivity – Anaphylaxis
Laboratory Abnormalities – Increased ALT, increased AST
Neurological – Peripheral sensory and motor neuropathy, seizures, coma
Ocular – Conjunctivitis, keratitis, lacrimation
Vascular – Phlebosclerosis, phlebitis/thrombophlebitis, hot flashes, thromboembolism
Other – Malaise/asthenia, fever, chills, weight gain
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Doxorubicin Hydrochloride Injection
Inhibitors of CYP3A4, CYP2D6, and P-gp
Concomitant use of doxorubicin hydrochloride with inhibitors of CYP3A4, CYP2D6, or P-glycoprotein (P-gp), increased concentrations of doxorubicin, which may increase the incidence and severity of adverse reactions of doxorubicin hydrochloride. Avoid concomitant use of Doxorubicin Hydrochloride Injection with inhibitors of CYP3A4, CYP2D6, or P-gp.
7.2 Concomitant Use of Trastuzumab
Concomitant use of trastuzumab and doxorubicin hydrochloride results in an increased risk of cardiac dysfunction. Avoid concomitant administration of Doxorubicin Hydrochloride Injection and trastuzumab [see Warnings and Precautions (5.1)].
Patients receiving doxorubicin after stopping treatment with trastuzumab may also be at an increased risk of developing cardiotoxicity. Trastuzumab may persist in the circulation for up to 7 months. Therefore, avoid anthracycline-based therapy for up to 7 months after stopping trastuzumab when possible. If anthracyclines are used before this time, carefully monitor cardiac function.
7.3 Concomitant Use of Dexrazoxane
Do not administer dexrazoxane as a cardioprotectant at the initiation of doxorubicin hydrochloride-containing chemotherapy regimens. In a randomized trial in women with metastatic breast cancer, initiation of dexrazoxane with doxorubicin hydrochloride-based chemotherapy resulted in a significantly lower tumor response rate (48% vs. 63%; p = 0.007) and shorter time to progression compared to doxorubicin hydrochloride-based chemotherapy alone.
7.4 Concomitant Use of 6-Mercaptopurine
Doxorubicin hydrochloride may potentiate 6-mercaptopurine-induced hepatotoxicity. In 11 patients with refractory leukemia treated with 6-mercaptopurine (500 mg/m2 intravenously daily for 5 days per cycle every 2–3 weeks) and doxorubicin hydrochloride (50 mg/m2 intravenous once per cycle every 2–3 weeks) alone or with vincristine and prednisone, all developed hepatic dysfunction manifested by increased total serum bilirubin, alkaline phosphatase and aspartate aminotransferase.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals and its mechanism of action, Doxorubicin Hydrochloride Injection can cause fetal harm when administered to a pregnant woman; avoid the use of Doxorubicin Hydrochloride Injection during the 1st trimester. Available human data do not establish the presence or absence of major birth defects and miscarriage related to the use of doxorubicin hydrochloride during the 2nd and 3rd trimesters. Doxorubicin hydrochloride was teratogenic and embryotoxic in rats and embryotoxic in rabbits when administered during organogenesis at doses approximately 0.07 times (based on body surface area) the recommended human dose of 60 mg/m2 (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Doxorubicin hydrochloride was teratogenic and embryotoxic at doses of 0.8 mg/kg/day (about 0.07 times the recommended human dose based on body surface area) when administered during the period of organogenesis in rats. Teratogenicity and embryotoxicity were also seen using discrete periods of treatment. The most susceptible was the 6- to 9-day gestation period at doses of 1.25 mg/kg/day and greater. Characteristic malformations included esophageal and intestinal atresia, tracheo-esophageal fistula, hypoplasia of the urinary bladder, and cardiovascular anomalies. Doxorubicin hydrochloride was embryotoxic (increase in embryo‑fetal deaths) and abortifacient at 0.4 mg/kg/day (about 0.07 times the recommended human dose based on body surface area) in rabbits when administered during the period of organogenesis.
8.2 Lactation
Risk Summary
Doxorubicin was measured in the milk of one lactating patient after therapy with 70 mg/m2 of doxorubicin hydrochloride given as a 15-minute intravenous infusion. The peak milk concentration at 24 hours after treatment was 4.4-fold greater than the corresponding plasma concentration. Doxorubicin was detectable in the milk up to 72 hours. There are no data on the effects of doxorubicin hydrochloride on the breastfed child or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with Doxorubicin Hydrochloride Injection and for 10 days after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating Doxorubicin Hydrochloride Injection.
Contraception
Females
Doxorubicin Hydrochloride Injection can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise female patients of reproductive potential to use highly effective contraception during treatment with Doxorubicin Hydrochloride Injection and for 6 months after treatment. [see Use in Specific Populations (8.1)].
Males
Doxorubicin hydrochloride may damage spermatozoa and testicular tissue, resulting in possible genetic fetal abnormalities. Due to the potential for genotoxicity, advise males with female partners of reproductive potential to use effective contraception during treatment with Doxorubicin Hydrochloride Injection and for 3 months after treatment [see Nonclinical Toxicology (13.1)]. Males with pregnant partners should use condoms during treatment and for at least 10 days after the final dose [see Nonclinical Toxicology (13.1), Use in Specific Populations (8.1)].
Infertility
Females
In females of reproductive potential, Doxorubicin hydrochloride may cause infertility and result in amenorrhea. Premature menopause can occur. Recovery of menses and ovulation is related to age at treatment [see Nonclinical Toxicology (13.1)].
Males
Doxorubicin hydrochloride may result in oligospermia, azoospermia, and permanent loss of fertility. Sperm counts have been reported to return to normal levels in some men. This may occur several years after the end of therapy [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Based on postmarketing reports, pediatric patients treated with doxorubicin hydrochloride are at risk for developing late cardiovascular dysfunction. Risk factors include young age at treatment (especially < 5 years), high cumulative doses and receipt of combined modality therapy. Long-term periodic cardiovascular monitoring is recommended for all pediatric patients who have received doxorubicin hydrochloride. Doxorubicin hydrochloride, as a component of intensive chemotherapy regimens administered to pediatric patients, may contribute to prepubertal growth failure and may also contribute to gonadal impairment, which is usually temporary.
There are no recommended dose adjustments based on age. Doxorubicin clearance was increased in patients aged 2 years to 20 years as compared to adults, while doxorubicin clearance was similar in infants less than 2 years as compared to adults [see Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Clinical experience in patients who were 65 years of age and older who received doxorubicin hydrochloride-based chemotherapy regimens for metastatic breast cancer showed no overall differences in safety and effectiveness compared with younger patients.
8.6 Hepatic Impairment
The clearance of doxorubicin was reduced in patients with elevated serum total bilirubin levels. Doxorubicin Hydrochloride Injection is contraindicated in patients with severe hepatic impairment (defined as Child Pugh Class C or serum bilirubin levels greater than 5 mg/dL) [see Contraindications (4)]. Reduce the dose of Doxorubicin Hydrochloride Injection in patients with serum total bilirubin levels greater than 1.2 mg/dL [See Dosage and Administration (2.4), Warnings and Precautions (5.5)].
-
10 OVERDOSAGE
Few cases of overdose have been described.
A 58-year-old man with acute lymphoblastic leukemia received 10-fold overdose of doxorubicin hydrochloride (300 mg/m2) in one day. He was treated with charcoal filtration, hemopoietic growth factor (G-CSF), proton pump inhibitor and antimicrobial prophylaxis. The patient suffered sinus tachycardia, grade 4 neutropenia and thrombocytopenia for 11 days, severe mucositis and sepsis. The patient recovered completely 26 days after the overdose.
A 17-year-old girl with osteogenic sarcoma received 150 mg of doxorubicin hydrochloride daily for 2 days (intended dose was 50 mg per day for 3 days). The patient developed severe mucositis on days 4–7 after the overdose and chills and pyrexia on day 7. The patient was treated with antibiotics and platelets and recovered 18 days after overdose.
-
11 DESCRIPTION
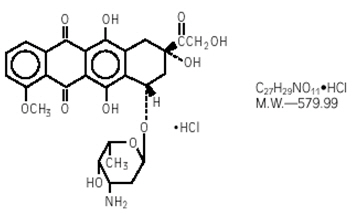
Doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor isolated from cultures of Streptomyces peucetius var. caesius. The chemical name of doxorubicin hydrochloride is 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxylacetyl)-1-methoxy-, hydrochloride (8S-cis)-. The chemical structure of doxorubicin hydrochloride is:
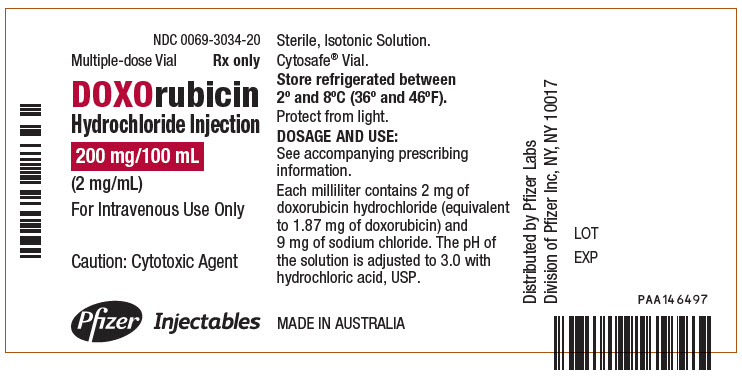
Doxorubicin Hydrochloride Injection, for intravenous use is a clear red, sterile, isotonic aqueous solution provided in vials containing 10 mg/5 mL doxorubicin hydrochloride (equivalent to 9.37 mg of doxorubicin free base), 20 mg/10 mL doxorubicin hydrochloride (equivalent to 18.74 mg of doxorubicin free base), 50 mg/25 mL doxorubicin hydrochloride (equivalent to 46.86 mg of doxorubicin free base), 150 mg/75 mL doxorubicin hydrochloride (140.58 mg of doxorubicin free base), or 200 mg/100 mL doxorubicin hydrochloride (equivalent to 187.4 mg of doxorubicin free base). The drug product has demonstrated inherent antimicrobial activity suitable for a multiple dose presentation. Each milliliter of solution contains 2 mg of doxorubicin hydrochloride and 9 mg of sodium chloride. The pH of the solution is adjusted to 3.0 with hydrochloric acid, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The cytotoxic effect of doxorubicin hydrochloride on malignant cells and its toxic effects on various organs are thought to be related to nucleotide base intercalation and cell membrane lipid binding activities of doxorubicin. Intercalation inhibits nucleotide replication and action of DNA and RNA polymerases. The interaction of doxorubicin with topoisomerase II to form DNA-cleavable complexes appears to be an important mechanism of doxorubicin hydrochloride cytocidal activity.
12.3 Pharmacokinetics
Pharmacokinetic studies conducted in patients with various types of tumors have shown that doxorubicin follows multiphasic disposition after intravenous injection. In four patients, doxorubicin demonstrated dose-independent pharmacokinetics across a dose range of 30 mg/m2 to 70 mg/m2.
Distribution
The distribution half-life is approximately 5 minutes. Steady-state distribution volume ranges from 809 L/m2 to 1214 L/m2. Binding of doxorubicin and its major metabolite, doxorubicinol, to plasma proteins is 75% and is independent of plasma concentration of doxorubicin up to 1.1 µg/mL.
Doxorubicin does not cross the blood brain barrier.
Elimination
Plasma clearance is ranges from 324 mL/min/m2 to 809 mL/min/m2. The terminal half-life is 20 hours to 48 hours.
Metabolism
Doxorubicin is a substrate of CYP3A4, CYP2D6, and P-gp.
Enzymatic reduction at the 7 position and cleavage of the daunosamine sugar yields aglycones which are accompanied by free radical formation, the local production of which may contribute to the cardiotoxic activity of doxorubicin hydrochloride.
Disposition of doxorubicinol in patients is formation rate limited, with the terminal half-life of doxorubicinol being similar to doxorubicin. The relative exposure of doxorubicinol, i.e., the ratio between the AUC of doxorubicinol and the AUC of doxorubicin is approximately 0.5.
Excretion
Plasma clearance is predominately by metabolism and biliary excretion. Approximately 40% of the dose appears in the bile in 5 days, while only 5% to 12% of the drug and its metabolites appear in the urine during the same time period. In urine, <3% of the dose was recovered as doxorubicinol over 7 days.
Specific Populations
Weight
Systemic clearance of doxorubicin is significantly reduced in obese women with ideal body weight greater than 130%. There was a significant reduction in clearance without any change in volume of distribution in obese patients when compared with normal patients with less than 115% ideal body weight.
Pediatric Patients
Following administration of doses ranging from 10 mg/m2 to 75 mg/m2 of doxorubicin hydrochloride to 60 patients ranging from 2 months to 20 years, doxorubicin clearance averaged 1443 ± 114 mL/min/m2. Further analysis demonstrated that clearance in 52 patients ranging from 2 to 20 years (1540 mL/min/m2) was increased compared with adults. However, clearance in infants younger than 2 years of age (813 mL/min/m2) was decreased compared with older patients (ranging from 2 to 20 years) and approached the range of clearance values determined in adults [see Use in Specific Populations (8.4)].
Sex
A published clinical study involving 6 men and 21 women with no prior anthracycline therapy reported a significantly higher median doxorubicin clearance in men compared to women (1088 mL/min/m2 versus 433 mL/min/m2). However, the terminal half-life of doxorubicin was longer in men compared to women (54 versus 35 hours).
Patients with Hepatic Impairment
The clearance of doxorubicin and doxorubicinol was reduced in patients with elevated serum total bilirubin concentrations [see Dosage and Administration (2.4), Warnings and Precautions (5.5)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Doxorubicin hydrochloride treatment can increase the risk of secondary malignancies based on postmarketing reports [see Warnings and Precautions (5.2)]. Doxorubicin hydrochloride was mutagenic in the in vitro Ames assay, and clastogenic in multiple in vitro assays (CHO cell, V79 hamster cell, human lymphoblast, and SCE assays) and the in vivo mouse micronucleus assay.
Doxorubicin hydrochloride decreased fertility in female rats at the doses of 0.05 and 0.2 mg/kg/day (approximately 0.005 and 0.02 times the recommended human dose, based on body surface area).
A single intravenous dose of 0.1 mg/kg doxorubicin hydrochloride (approximately 0.01 times the recommended human dose based on body surface area) was toxic to male reproductive organs in animal studies, producing testicular atrophy, diffuse degeneration of the seminiferous tubules, and oligospermia/hypospermia in rats. Doxorubicin hydrochloride induces DNA damage in rabbit spermatozoa and dominant lethal mutations in mice.
-
14 CLINICAL STUDIES
14.1 Adjuvant Breast Cancer
The efficacy of doxorubicin hydrochloride-containing regimens for the post-operative, adjuvant treatment of surgically resected breast cancer was evaluated in a meta-analysis conducted by the Early Breast Cancer Trialists Collaborative Group (EBCTCG). The EBCTCG meta-analyses compared cyclophosphamide, methotrexate, and fluorouracil (CMF) to no chemotherapy (19 trials including 7523 patients) and doxorubicin hydrochloride-containing regimens with CMF as an active control (6 trials including 3510 patients). Data from the meta-analysis of trials comparing CMF to no therapy were used to establish the historical treatment effect size for CMF regimens. The major efficacy outcome measures were disease-free survival (DFS) and overall survival (OS).
Of the 3510 women (2157 received doxorubicin hydrochloride-containing regimens and 1353 received CMF treatment) with early breast cancer involving axillary lymph nodes included in the six trials from the meta-analyses, approximately 70% were premenopausal and 30% were postmenopausal.
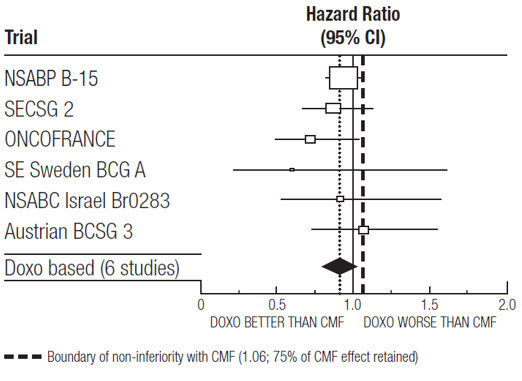
At the time of the meta-analysis, 1745 first recurrences and 1348 deaths had occurred. The analyses demonstrated that doxorubicin hydrochloride-containing regimens retained at least 75% of the historical CMF adjuvant effect on DFS with a hazard ratio (HR) of 0.91 (95% CI: 0.82, 1.01) and on OS with a HR of 0.91 (95% CI: 0.81, 1.03). Efficacy results are provided in Table 3 and Figures 1 and 2.
Table 3. Summary of Randomized Trials Comparing Doxorubicin Hydrochloride-Containing Regimens Versus CMF in Meta-Analysis Study
(starting year)Regimens No. of Cycles No. of Patients Doxorubicin Hydrochloride-Containing Regimens vs. CMF
HR* (95% CI)DFS OS Abbreviations: DFS = disease free survival; OS = overall survival; AC = doxorubicin hydrochloride, cyclophosphamide; AVbCMF = doxorubicin hydrochloride, vinblastine, cyclophosphamide, methotrexate, fluorouracil; CMF = cyclophosphamide, methotrexate, fluorouracil; CMFVA = cyclophosphamide, methotrexate, fluorouracil, vincristine, doxorubicin hydrochloride; FAC = fluorouracil, doxorubicin hydrochloride, cyclophosphamide; FACV = fluorouracil, doxorubicin hydrochloride, cyclophosphamide, vincristine; HR = hazard ratio; CI = confidence interval - *
- Hazard ratio of less than 1 indicates that the treatment with doxorubicin hydrochloride-containing regimens is associated with lower risk of disease recurrences or death compared to the treatment with CMF.
- †
- Includes pooled data from patients who received either AC alone for 4 cycles, or who were treated with AC for 4 cycles followed by 3 cycles of CMF.
- ‡
- Patients received alternating cycles of AVb and CMF.
NSABP B-15
(1984)AC
4
1562†
0.93 (0.82, 1.06)
0.97 (0.83, 1.12)
CMF
6
776
SECSG 2
(1976)FAC
6
260
0.86 (0.66, 1.13)
0.93 (0.69, 1.26)
CMF
6
268
ONCOFRANCE
(1978)FACV
12
138
0.71 (0.49, 1.03)
0.65 (0.44, 0.96)
CMF
12
113
SE Sweden BCG A
(1980)AC
6
21
0.59 (0.22, 1.61)
0.53 (0.21, 1.37)
CMF
6
22
NSABC Israel Br0283
(1983)AVbCMF‡
4
655
0.91 (0.53, 1.57)
0.88 (0.47, 1.63)
CMF
6
50
Austrian BCSG 3
(1984)CMFVA
6
121
1.07 (0.73, 1.55)
0.93 (0.64, 1.35)
CMF
8
124
Combined Studies
Doxorubicin Hydrochloride-Containing Regimen
2157
0.91 (0.82, 1.01)
0.91 (0.81, 1.03)
CMF
1353
Figure 1. Meta-analysis of Disease-Free Survival
Figure 2. Meta-analysis of Overall Survival
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Doxorubicin Hydrochloride Injection, is available as follows:
Doxorubicin Hydrochloride Injection is a sterile, isotonic solution, available in single-dose polypropylene (CYTOSAFE)® vials, and multiple-dose polypropylene (CYTOSAFE)® vials:
Unit of Sale
Total Strength/Total Volume
(Concentration)
NDC 0069-3030-20
Carton of 1 Single-dose Vial
10 mg/5 mL
(2 mg/mL)
NDC 0069-3031-20
Carton of 1 Single-dose Vial
20 mg/10 mL
(2 mg/mL)
NDC 0069-3032-20
Carton of 1 Single-dose Vial
50 mg/25 mL
(2 mg/mL)
NDC 0069-3033-20
Carton of 1 Multiple-dose Vial
150 mg/75 mL
(2 mg/mL)
NDC 0069-3034-20
Carton of 1 Multiple-dose Vial
200 mg/100 mL
(2 mg/mL)
Retain in carton until contents are used. For single-dose vials, discard unused portion.
Doxorubicin Hydrochloride Injection is a sterile, isotonic solution, available in single-dose ONCO-TAIN® glass vials, and multiple-dose ONCO-TAIN® glass vials:
Unit of Sale
Total Strength/Total Volume
(Concentration)
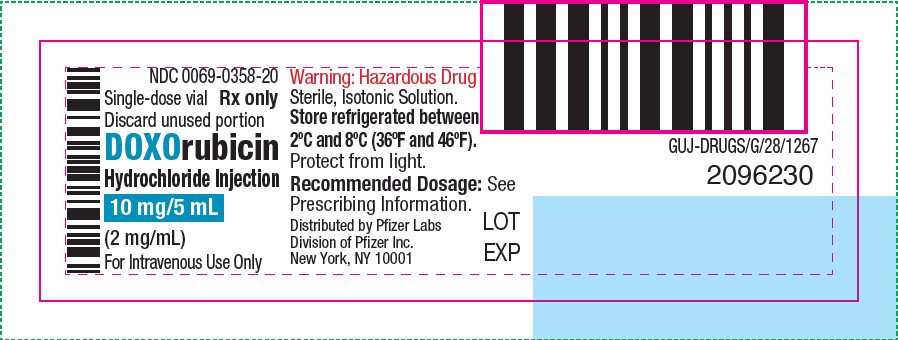
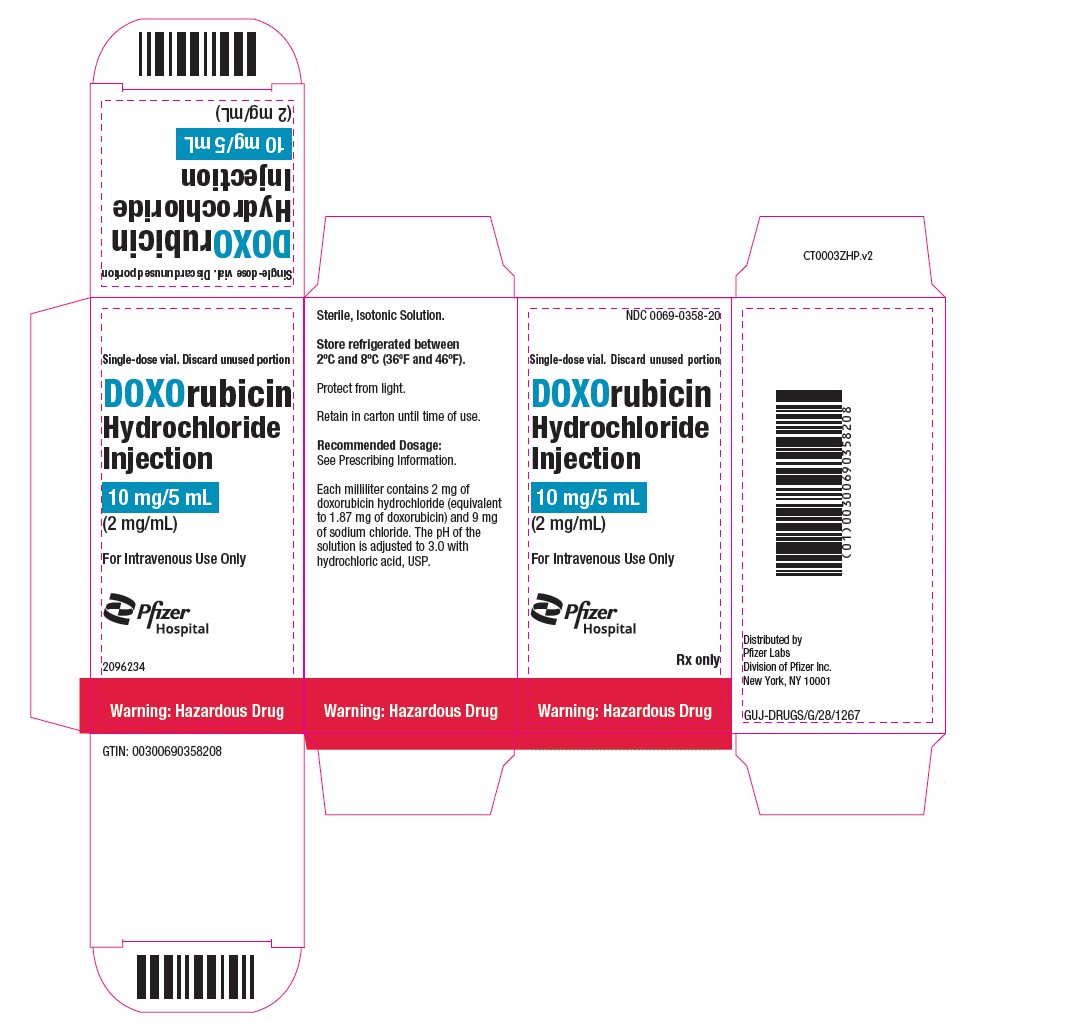
NDC 0069-0358-20
Carton of 1 Single-dose Vial
10 mg/5 mL
(2 mg/mL)
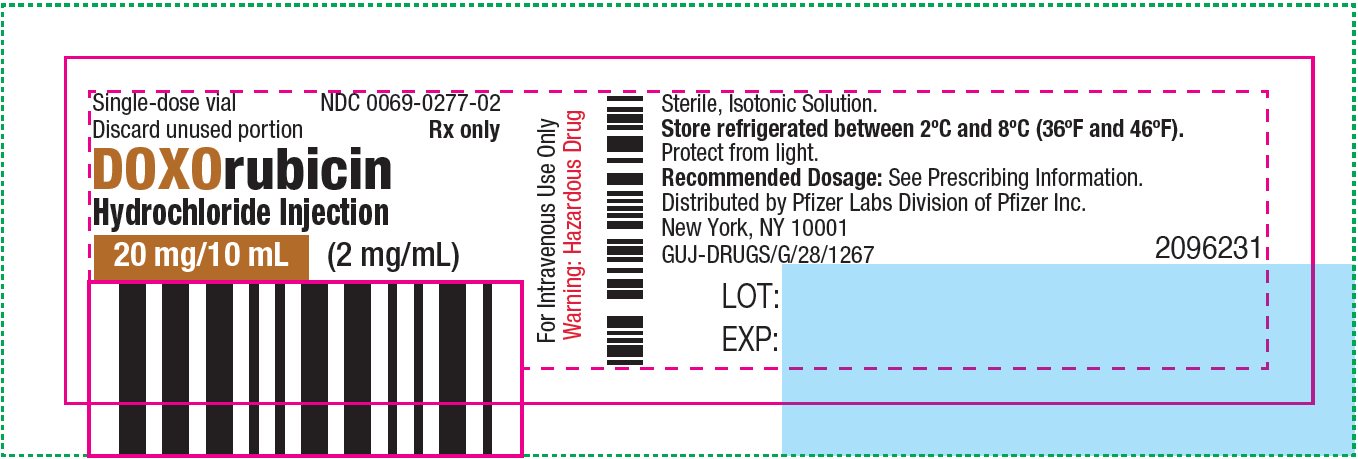
NDC 0069-0277-02
Carton of 1 Single-dose Vial
20 mg/10 mL
(2 mg/mL)
NDC 0069-0343-02
Carton of 1 Single-dose Vial
50 mg/25 mL
(2 mg/mL)
NDC 0069-1542-20
Carton of 1 Multiple-dose Vial
200 mg/100 mL
(2 mg/mL)
ONCO-TAIN® is the vial external protection system.
Retain in carton until contents are used. For single-dose ONCO-TAIN® glass vials, discard unused portion.
Storage
Store all vials at 2°C to 8°C (36°F to 46°F). Protect from light.
Storage of Doxorubicin Hydrochloride Injection under refrigerated conditions can result in the formation of a gelled product. Place gelled product at room temperature [15ºC to 30ºC (59ºF to 86ºF)] for 2 to 4 hours to return the product to a slightly viscous, mobile solution.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Cardiomyopathy
Advise patients that Doxorubicin Hydrochloride Injection can cause irreversible myocardial damage and to contact a healthcare provider for symptoms of heart failure during or after treatment [see Warnings and Precautions (5.1)].
Secondary Malignancy
Advise patients of the increased risk of treatment-related leukemia [see Warnings and Precautions (5.2)].
Myelosuppression
Advise patients that Doxorubicin Hydrochloride Injection can reduce the absolute neutrophil count resulting in an increased risk of infection and to contact a healthcare provider for new onset fever or symptoms of infection [see Warnings and Precautions (5.4)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus, and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with Doxorubicin Hydrochloride Injection and for 6 months after treatment [see Warnings and Precautions (5.8), Use in Specific Populations (8.3)].
Advise patients that Doxorubicin Hydrochloride Injection may induce chromosomal damage in sperm, which may lead to loss of fertility and offspring with birth defects. Advise males with female partners of reproductive potential to use effective contraception during treatment with Doxorubicin Hydrochloride Injection and for 3 months after treatment [see Warnings and Precautions (5.8), Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Advise males with pregnant partners to use condoms during treatment with Doxorubicin Hydrochloride Injection and for at least 10 days after the final dose [see Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with Doxorubicin Hydrochloride Injection and for 10 days after the final dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of the potential loss of fertility from Doxorubicin Hydrochloride Injection [see Use in Specific Populations (8.3)].
Gastrointestinal and Dermatologic Adverse Reactions
Advise patients that Doxorubicin Hydrochloride Injection can cause nausea, vomiting, diarrhea, mouth/oral pain and sores and to contact a healthcare provider should they develop any severe symptoms that prevent them from eating and drinking [see Adverse Reactions (6)]. Advise patients that Doxorubicin Hydrochloride Injection can cause alopecia [see Adverse Reactions (6.1)].
-
SPL UNCLASSIFIED SECTION
This product’s labeling may have been updated. For the most current prescribing information, please visit www.pfizer.com.
LAB-0073-19.0
-
PATIENT PACKAGE INSERT
Patient Information
DOXORUBICIN (dok-suh-roo-buh-sin) HYDROCHLORIDE
injection, for intravenous useWhat is the most important information I should know about Doxorubicin?
Doxorubicin may cause serious side effects including:
- •
-
Heart muscle problems. Doxorubicin can cause heart muscle damage that may lead to heart failure. Heart failure means your heart does not pump blood well. Heart failure is irreversible in some cases and can lead to death. Heart failure can happen during your treatment with Doxorubicin or months to years after stopping treatment. Your risk of heart muscle damage increases with higher total amounts of Doxorubicin that you receive in your lifetime. Your risk of heart failure is higher if you:
- o
- have other heart problems
- o
- have had or are currently receiving radiation therapy to your chest
- o
- have had treatment with certain other anti-cancer medicines
- o
- take other medicines that can have severe side effects on your heart
Tell your healthcare provider if you get any of these symptoms of heart failure during or after treatment with Doxorubicin:
- o
- extreme tiredness or weakness
- o
- shortness of breath
- o
- fast heartbeat
- o
- swelling of your feet and ankles
Your healthcare provider will do tests to check the strength of your heart muscle before, during, and after your treatment with Doxorubicin.
- •
- Heart rhythm problems. Doxorubicin can cause serious heart rhythm problems that may lead to death. This can happen during your infusion, within a few hours after your infusion or anytime during treatment with Doxorubicin. Tell your healthcare provider if you get any symptoms of heart rhythm problems, such as feeling as if your heart is beating fast, irregular or slow, or you feel lightheaded, dizzy, short of breath, chest discomfort or you faint.
- •
- Risk of new cancers. You may have an increased risk of developing certain blood cancers called acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) after treatment with Doxorubicin. Talk with your healthcare provider about your risk of developing new cancers if you receive Doxorubicin.
- •
- Skin damage at or near the vein where Doxorubicin is given. Doxorubicin can damage the skin if it leaks out of the vein and might cause blisters, skin sores or severe tissue damage, which may require skin grafts. Tell your healthcare provider if you get burning or stinging during your infusion.
- •
- Decreased blood cell counts. Doxorubicin can cause a decrease in neutrophils (a type of white blood cell important in fighting bacterial infections) and platelets (important for clotting and to control bleeding). This may lead to a serious infection, the need for blood transfusions, treatment in a hospital or death. Your healthcare provider will check your blood cell counts before each infusion and during treatment with Doxorubicin. Call your healthcare provider right away if you get a fever (temperature of 100.4°F or higher) or chills with shivering.
What is Doxorubicin?
Doxorubicin is a prescription medicine used to treat certain types of cancers. Doxorubicin may be used alone or along with other anti-cancer medicines.
Do not receive Doxorubicin if:
- •
- you have had a recent heart attack (within the past 4 to 6 weeks) or have severe heart problems.
- •
- your blood cell counts (platelets, red blood cells, and white blood cells) are very low because of prior chemotherapy.
- •
- you have severe liver problems.
- •
- you have had a severe allergic reaction to Doxorubicin.
Before you receive Doxorubicin, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have heart problems including heart failure.
- •
- are currently receiving radiation therapy or plan to receive radiation to the chest.
- •
- have liver problems.
- •
- have had an allergic reaction to doxorubicin.
- •
- are pregnant or plan to become pregnant. Doxorubicin can harm your unborn baby. You should not become pregnant during treatment with Doxorubicin. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
Females who are able to become pregnant:- o
- Your healthcare provider will check to see if you are pregnant before you start treatment with Doxorubicin.
- o
- You should use effective birth control (contraception) during treatment with Doxorubicin and for 6 months after treatment.
-
Males:
- o
- Doxorubicin can affect your sperm and could cause birth defects.
- o
- If you have a female partner who can become pregnant, you should use effective birth control during treatment with Doxorubicin and for 3 months after treatment.
- o
- If you have a pregnant partner, you should use condoms during treatment with Doxorubicin and for at least 10 days after the final dose.
- o
- Talk to your healthcare provider about birth control methods that may be right for you.
- •
- are breastfeeding or plan to breastfeed. Doxorubicin can pass into your breast milk. Do not breastfeed during treatment with Doxorubicin and for 10 days after the final dose. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive Doxorubicin?
- •
- Doxorubicin will be given to you into your vein through an intravenous (IV) line.
- •
- Your healthcare provider will do blood tests to check for side effects during treatment with Doxorubicin.
- •
- Your healthcare provider may stop your treatment, change the timing of your treatment, or change the dose of your treatment if you have certain side effects while receiving Doxorubicin.
What are the possible side effects of Doxorubicin?
Doxorubicin may cause serious side effects, including:
The most common side effects of Doxorubicin include:
- •
- total hair loss (alopecia). Your hair may re-grow after your treatment.
- •
- nausea
- •
- vomiting
Other side effects:
- •
- Red colored urine. You may have red colored urine for 1 to 2 days after your infusion of Doxorubicin. This is normal. Tell your healthcare provider if it does not stop in a few days, or if you see what looks like blood or blood clots in your urine.
- •
- Call your healthcare provider if you have severe symptoms that prevent you from eating or drinking, such as:
- o
- nausea
- o
- vomiting
- o
- diarrhea
- o
- mouth pain or sores
Doxorubicin may cause fertility problems in males. This could affect your ability to father a child. Talk to your healthcare provider if this is a concern for you.
Doxorubicin may cause fertility problems in females. Your periods (menstrual cycle) may completely stop when you receive Doxorubicin. Your periods may or may not return following treatment. Early menopause has also happened. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Doxorubicin.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Doxorubicin.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.You can ask your pharmacist or healthcare provider for information about Doxorubicin that is written for health professionals.
What are the ingredients in Doxorubicin?
Active ingredient: doxorubicin hydrochloride
Inactive ingredients for Doxorubicin Hydrochloride Injection: sodium chloride, and hydrochloric acid, USP.

LAB-0436-5.0
For more information, call 1-800-438-1985 or visit www.pfizer.com.This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 7/2024
- PRINCIPAL DISPLAY PANEL - 10 mg/5 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 10 mg/5 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 20 mg/10 mL Vial Label
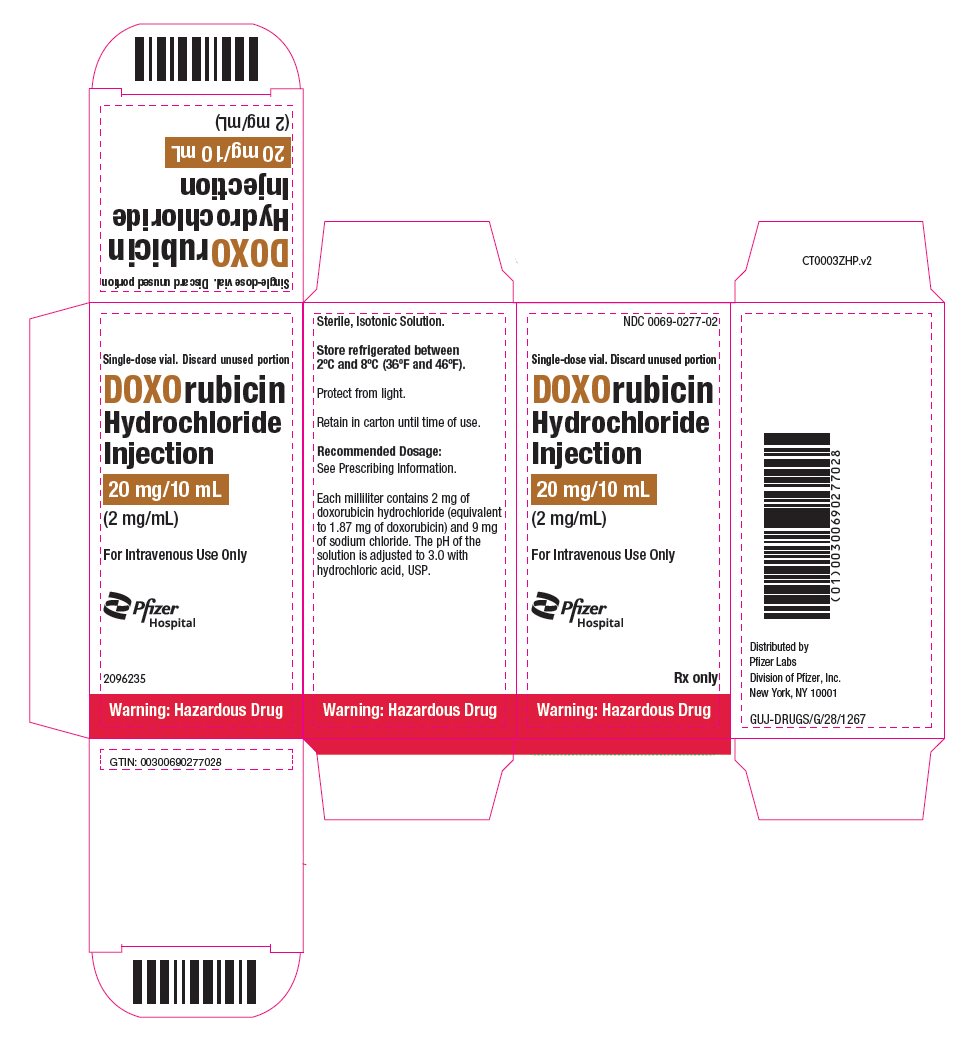
- PRINCIPAL DISPLAY PANEL - 20 mg/10 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial Label
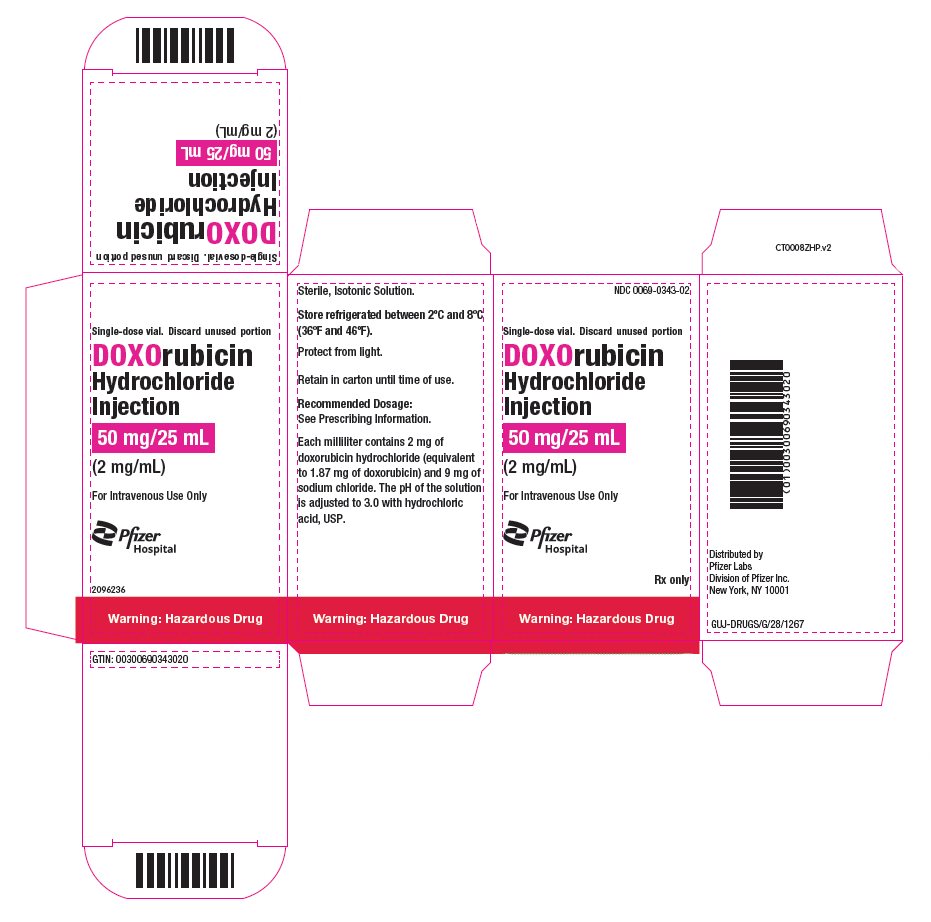
- PRINCIPAL DISPLAY PANEL - 50 mg/25 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 150 mg/75 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 150 mg/75 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 200 mg/100 mL Vial Carton
- PRINCIPAL DISPLAY PANEL – 10 mg/5 mL Vial ONCO-TAIN® Label
- PRINCIPAL DISPLAY PANEL – 10 mg/5 mL Vial ONCO-TAIN® Carton
- PRINCIPAL DISPLAY PANEL – 20 mg/10 mL Vial ONCO-TAIN® Label
- PRINCIPAL DISPLAY PANEL – 20 mg/10 mL Vial ONCO-TAIN® Carton
- PRINCIPAL DISPLAY PANEL – 50 mg/25 mL Vial ONCO-TAIN® Label
- PRINCIPAL DISPLAY PANEL – 50 mg/25 mL Vial ONCO-TAIN® Carton
- PRINCIPAL DISPLAY PANEL – 200 mg/100 mL Vial ONCO-TAIN® Label
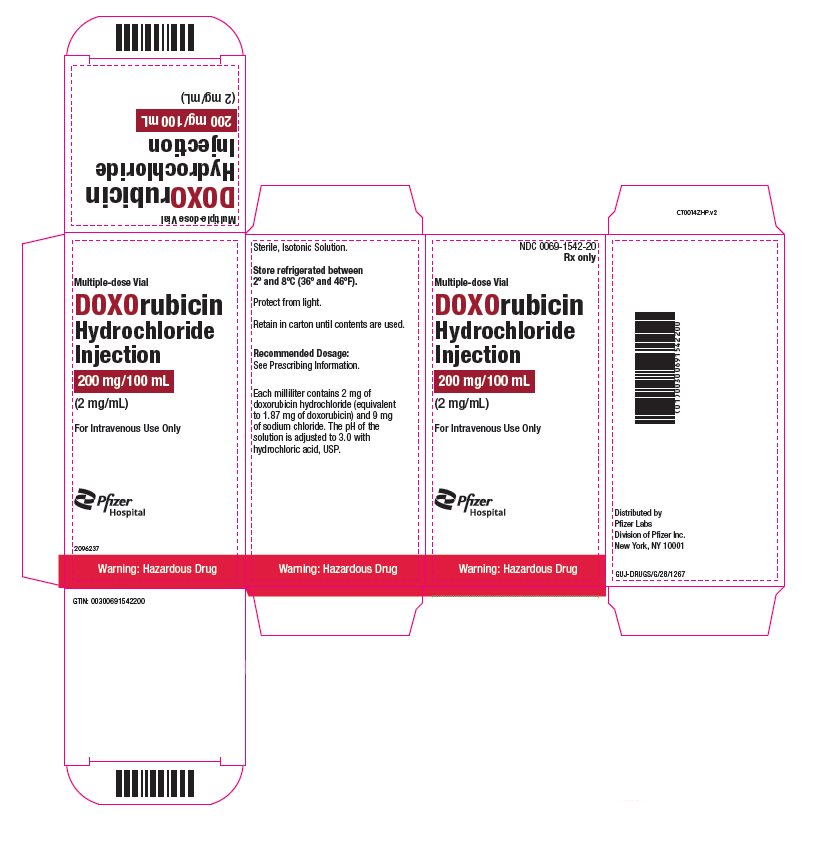
- PRINCIPAL DISPLAY PANEL – 200 mg/100 mL Vial ONCO-TAIN® Carton
-
INGREDIENTS AND APPEARANCE
DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-3030 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-3030-20 1 in 1 CARTON 12/23/1987 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 12/23/1987 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-3031 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-3031-20 1 in 1 CARTON 12/23/1987 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 12/23/1987 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-3032 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-3032-20 1 in 1 CARTON 12/23/1987 1 25 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 12/23/1987 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-3033 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-3033-20 1 in 1 CARTON 12/23/1987 07/31/2024 1 75 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 12/23/1987 07/31/2024 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-3034 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-3034-20 1 in 1 CARTON 12/23/1987 1 100 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 12/23/1987 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0358 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0358-20 1 in 1 CARTON 08/19/2024 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 08/19/2024 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0277 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0277-02 1 in 1 CARTON 08/19/2024 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 08/19/2024 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0343 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0343-02 1 in 1 CARTON 05/09/2024 05/09/2024 1 25 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 05/09/2024 05/09/2024 DOXORUBICIN HYDROCHLORIDE
doxorubicin hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-1542 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXORUBICIN HYDROCHLORIDE (UNII: 82F2G7BL4E) (DOXORUBICIN - UNII:80168379AG) DOXORUBICIN HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1542-20 1 in 1 CARTON 07/22/2024 1 100 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050629 07/22/2024 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525)