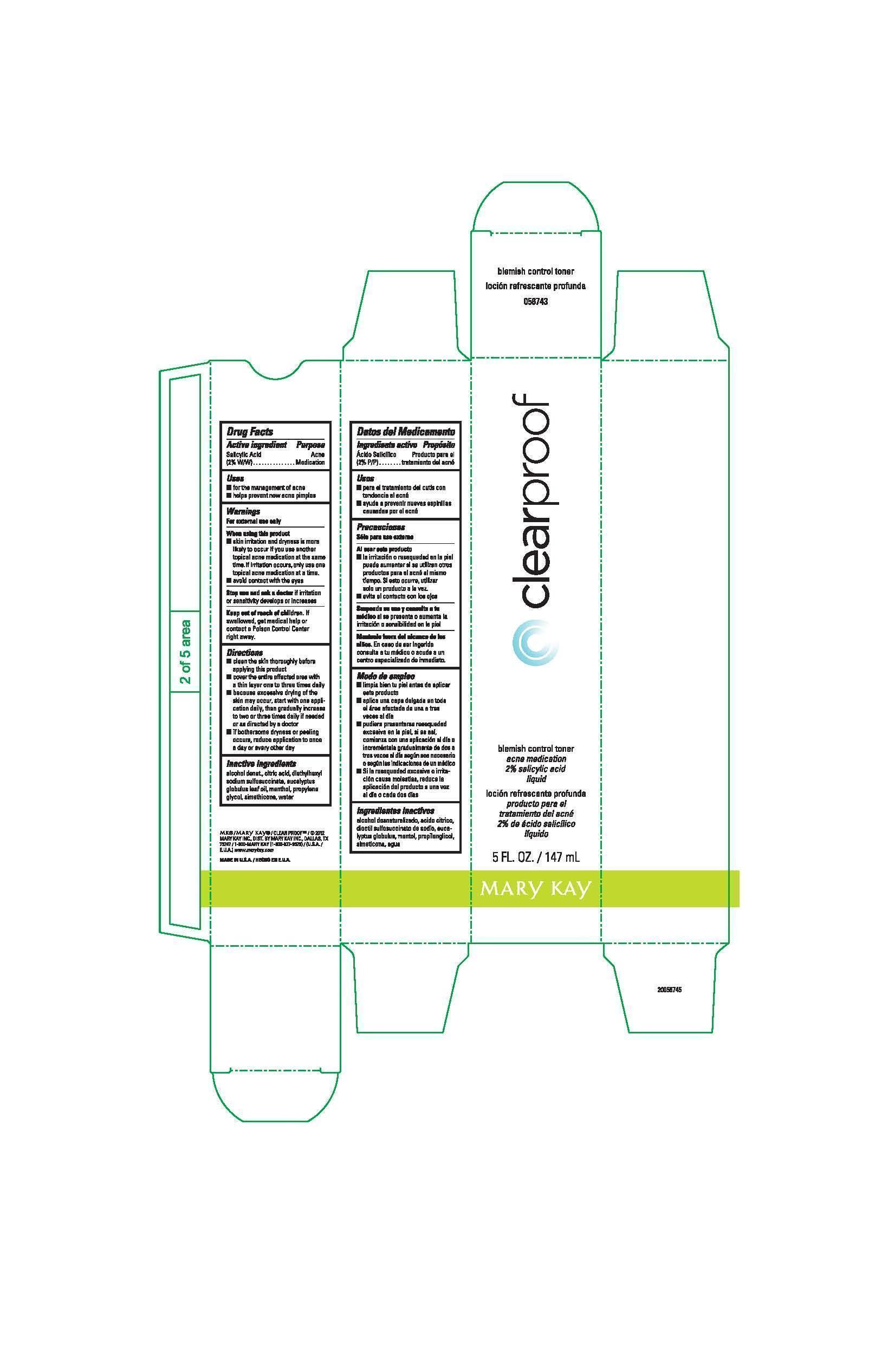
Label: CLEAR PROOF BLEMISH CONTROL TONER ACNE MEDICATION- salicylic acid liquid
- NDC Code(s): 51531-6743-5, 51531-6743-9
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Uses
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Inactive ingredients
- Principal Display Panel - 147 mL bottle
-
INGREDIENTS AND APPEARANCE
CLEAR PROOF BLEMISH CONTROL TONER ACNE MEDICATION
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-6743 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DOCUSATE SODIUM (UNII: F05Q2T2JA0) EUCALYPTUS OIL (UNII: 2R04ONI662) MENTHOL (UNII: L7T10EIP3A) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-6743-5 1 in 1 CARTON 08/15/2013 1 147 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:51531-6743-9 26 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/15/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/15/2013 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Port Jervis Laboratories Inc. 001535103 manufacture(51531-6743) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-6743)