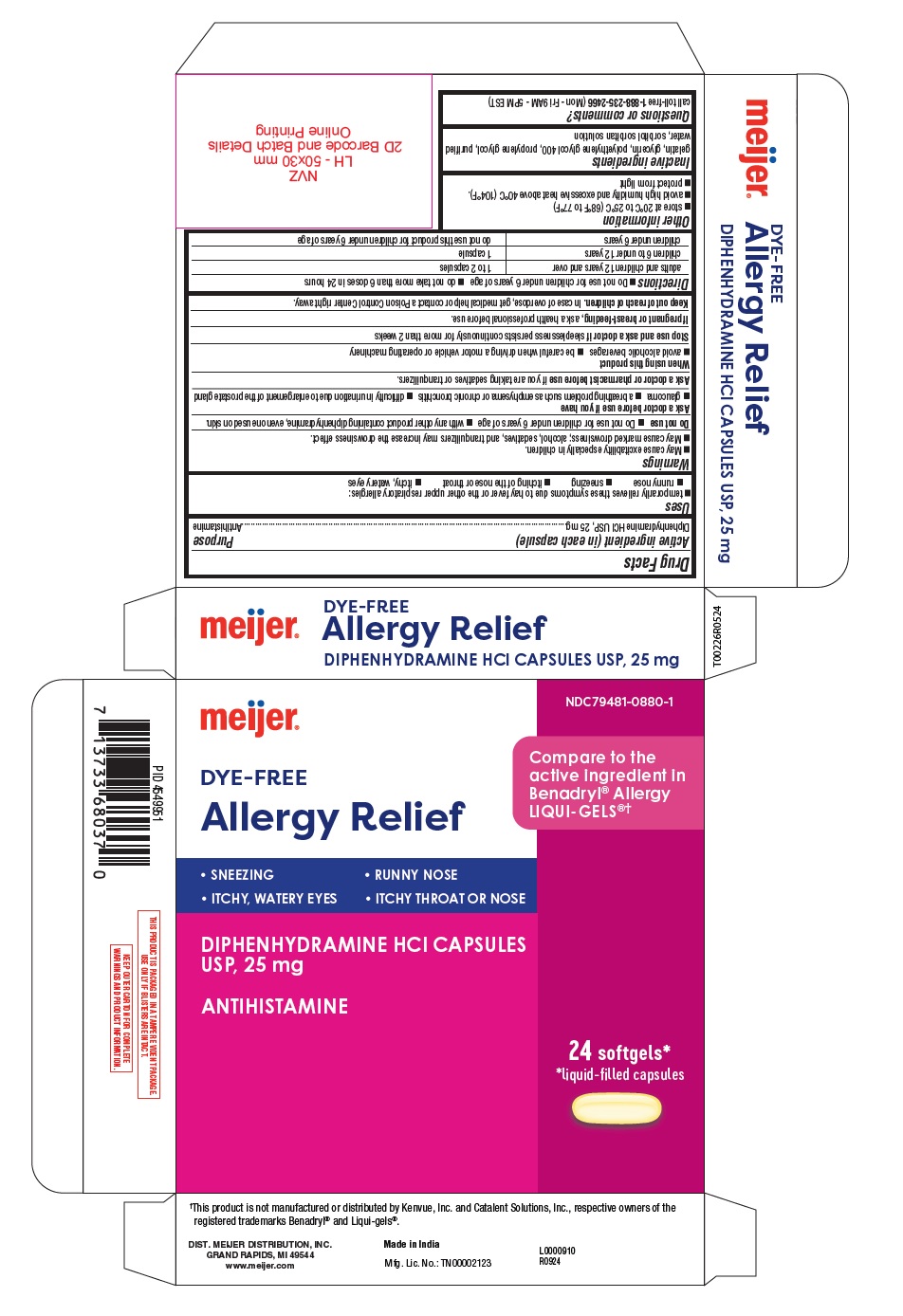
Label: ALLERGY RELIEF- diphenhydramine hcl capsule, liquid filled
- NDC Code(s): 79481-0880-1
- Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
THIS PRODUCT IS PACKAGED IN A TAMPER EVIDENT PACKAGE.
USE ONLY IF BLISTERS ARE INTACT.KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
†This product is not manufactured or distributed by Kenvue, Inc. and Catalent Solutions, Inc., respective owners of the
registered trademarks Benadryl® and Liqui-gels®.DIST. MEIJER DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.comMade in India
Mfg. Lic. No.: TN00002123R0924
L0000910 - 24's count
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hcl capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0880 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SORBITAN (UNII: 6O92ICV9RU) GELATIN (UNII: 2G86QN327L) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white (Clear) Score no score Shape CAPSULE Size 17mm Flavor Imprint Code DF25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0880-1 2 in 1 CARTON 11/26/2024 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/26/2024 Labeler - Meijer Distribution Inc (006959555) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations SOFTGEL HEALTHCARE PRIVATE LIMITED 675584180 manufacture(79481-0880)