Label: PROGESTERONE capsule
- NDC Code(s): 65162-807-10, 65162-807-50, 65162-808-10, 65162-808-50
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER AND PROBABLE DEMENTIA FOR ESTROGEN PLUS PROGESTIN THERAPY
Cardiovascular Disorders and Probable Dementia
Estrogens plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia (see CLINICAL STUDIES and WARNINGS, Cardiovascular disorders and Probable dementia).
The Women’s Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of deep vein thrombosis, pulmonary embolism, stroke and myocardial infarction in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo (see CLINICAL STUDIES and WARNINGS, Cardiovascular disorders).
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women (see CLINICAL STUDIES and WARNINGS, Probable dementia and PRECAUTIONS, Geriatric Use).
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer (see CLINICAL STUDIES and WARNINGS, Malignant neoplasms, Breast Cancer).
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins.
Progestins with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Close -
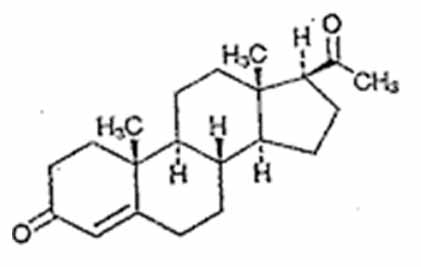
DESCRIPTIONProgesterone capsules contain progesterone, USP for oral administration. Progesterone, USP has a molecular weight of 314.47 and a molecular formula of C21H30O2. Progesterone, USP (pregn-4-ene-3 ...
-
CLINICAL PHARMACOLOGYProgesterone capsules are an oral dosage form of progesterone which is chemically identical to progesterone of ovarian origin. Pharmacokinetics - A. Absorption - After oral administration of ...
-
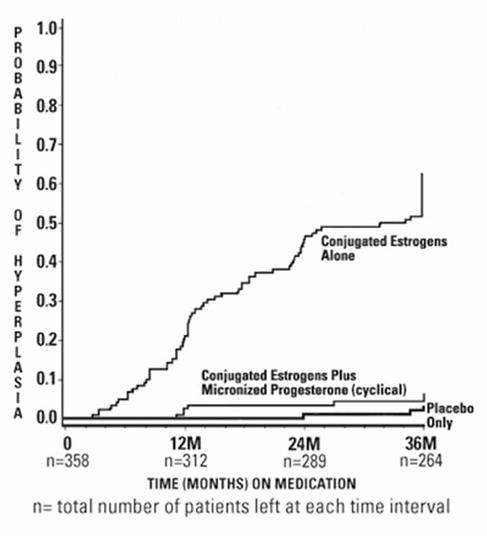
CLINICAL STUDIESEffects on the endometrium - In a randomized, double-blind clinical trial, 358 postmenopausal women, each with an intact uterus, received treatment for up to 36 months. The treatment groups were ...
-
INDICATIONS AND USAGEProgesterone capsules are indicated for use in the prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving conjugated estrogens tablets. They are also ...
-
CONTRAINDICATIONSProgesterone capsules should not be used in women with any of the following conditions: 1. Progesterone capsules should not be used in patients with known hypersensitivity to its ...
-
WARNINGSSee BOXED WARNING. 1. Cardiovascular disorders - An increased risk of pulmonary embolism, deep vein thrombosis (DVT), stroke and myocardial infarction has been reported with estrogen plus ...
-
PRECAUTIONSA. General - 1. Addition of a progestin when a woman has not had a hysterectomy - Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily ...
-
ADVERSE REACTIONSSee BOXED WARNING, WARNINGS and PRECAUTIONS. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be ...
-
OVERDOSAGENo studies on overdosage have been conducted in humans. In the case of overdosage, progesterone capsules should be discontinued and the patient should be treated symptomatically.
-
DOSAGE AND ADMINISTRATIONPrevention of Endometrial Hyperplasia - Progesterone capsules should be given as a single daily dose at bedtime, 200 mg orally for 12 days sequentially per 28-day cycle, to a postmenopausal woman ...
-
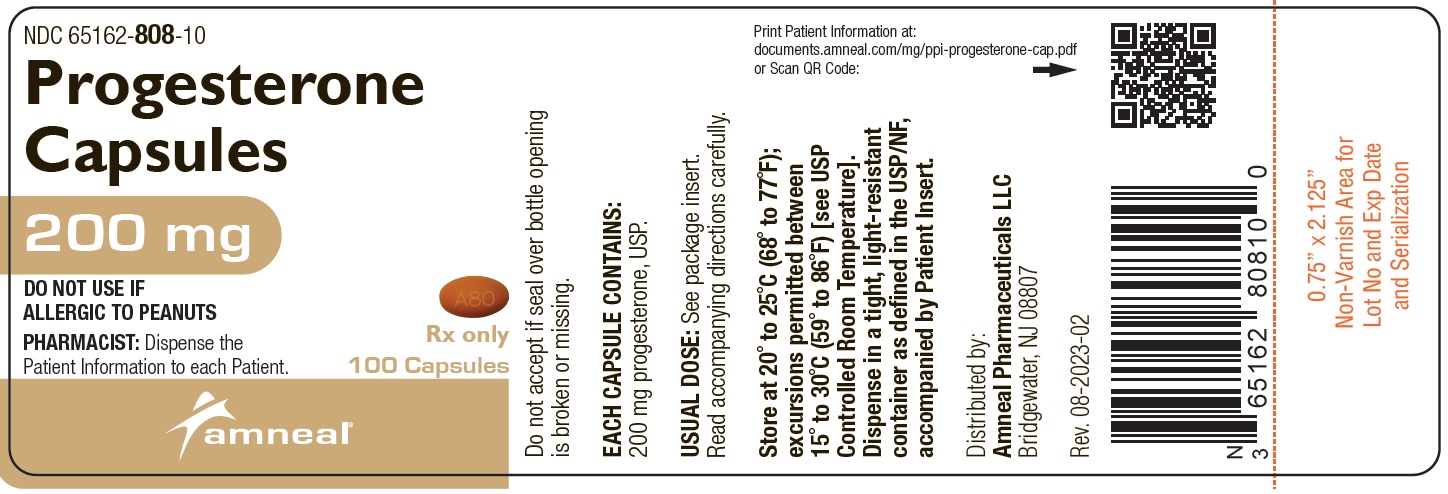
HOW SUPPLIEDProgesterone capsules, 100 mg, are supplied as round, light orange soft gelatin capsules, printed with “A87”. They are available as follows: Bottles of 100: NDC ...
-
PATIENT INFORMATION
Progesterone (proe-JESS-te-rone) Capsules (progesterone, USP), 100 mg and 200 mg - Rx only - Read this PATIENT INFORMATION before you start taking progesterone capsules and read what you get ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information