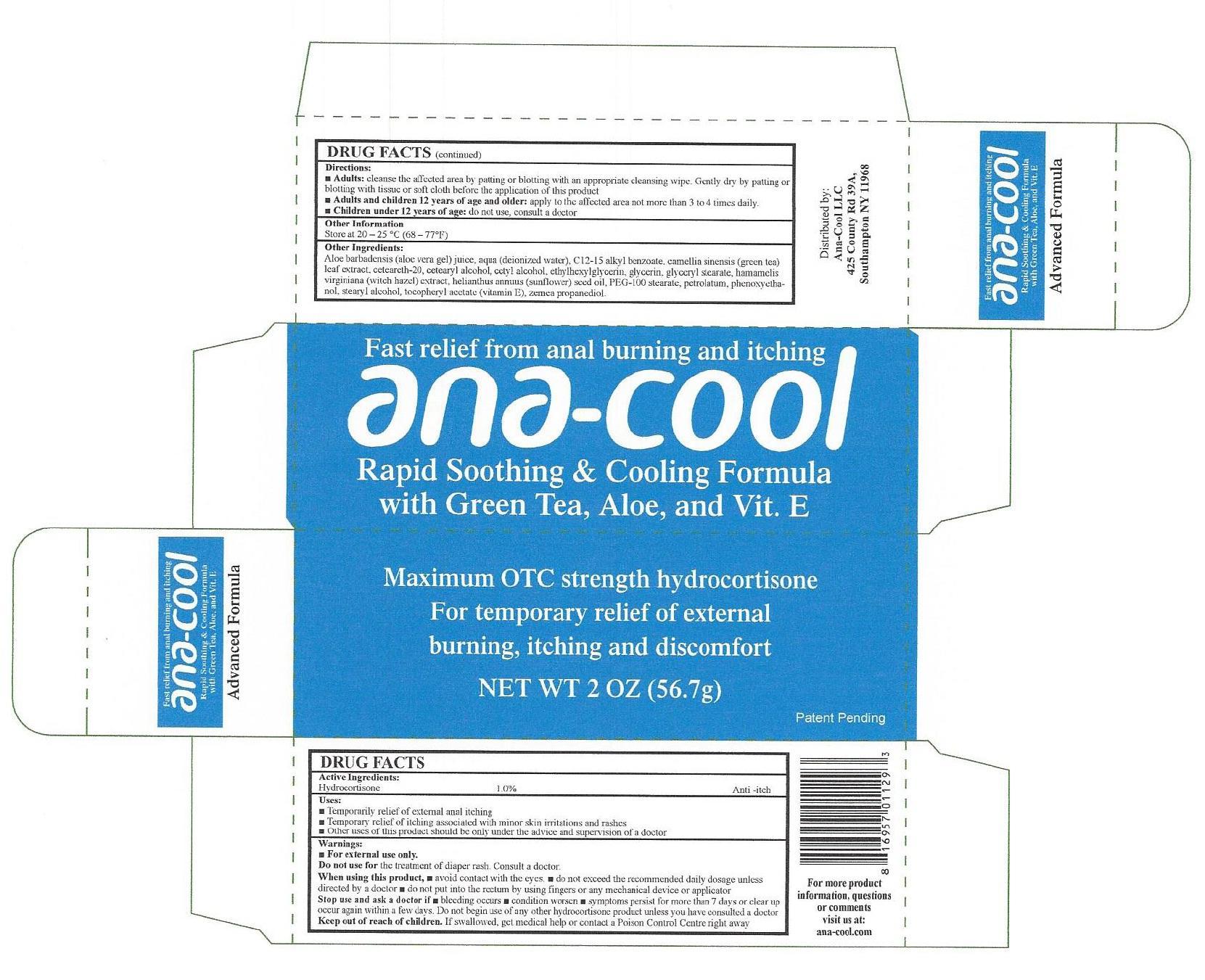
Label: ANA COOL RAPID SOOTHING COOLING FORMULA- hydrocortisone cream
- NDC Code(s): 70288-714-01
- Packager: Ana-Cool, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredients:
- Uses:
-
Warnings:
- For external use only.
When using this product,
• avoid contact with the eyes • do not exceed the recommended daily dosage unless directed by a doctor • do not put into the rectum by using fingers or any mechanical device or applicator
-
Directions:
- cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with tissue or soft cloth before the applicationof this product Adults:
- apply to the affected area not more than 3 to 4 times daily. Adults and children 12 years of age and older:
- do not use, consult a doctor Children under 12 years of age:
- Other Information
-
Other Ingredients:
Aloe barbadensis (aloe vera gel) juice, aqua (deionized water), C12-15 alkyl benzoate, camellia sinensis (green tea) leaf extract, cetearcth-20, cetearyl alcohol, cetyl alcohol, ethylhexylglycerin, glycerin, glyceryl stearate, hamamelis virginiana (witch hazel) extract, helianthus annuus (sunflower) seed oil, PEG-100 stearate, petrolatum, phenoxyethanol, stearyl alcohol, tocopheryl acetate (vitamin E), zemea propanediol.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ANA COOL RAPID SOOTHING COOLING FORMULA
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70288-714 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ALCOHOL (UNII: 936JST6JCN) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) SUNFLOWER OIL (UNII: 3W1JG795YI) ALOE VERA LEAF (UNII: ZY81Z83H0X) PEG-100 STEARATE (UNII: YD01N1999R) PETROLATUM (UNII: 4T6H12BN9U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70288-714-01 1 in 1 BOX 11/25/2015 1 56.7 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/25/2015 Labeler - Ana-Cool, LLC (091452072)