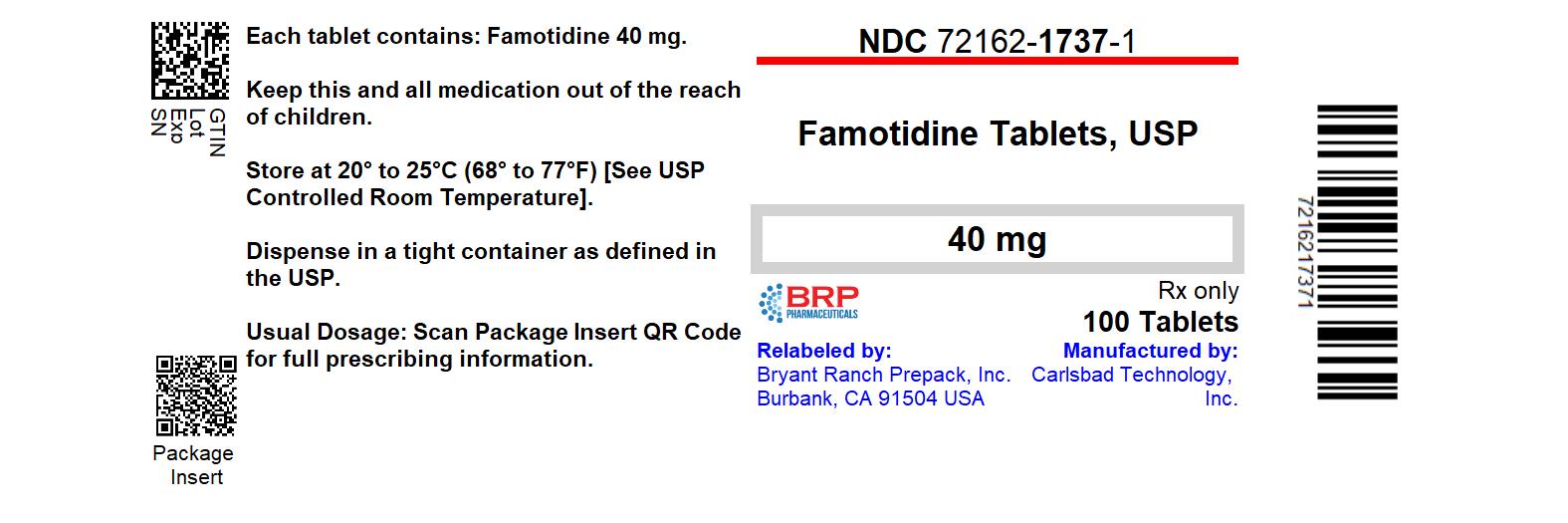
Label: FAMOTIDINE tablet
- NDC Code(s): 72162-1737-0, 72162-1737-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 61442-122
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FAMOTIDINE TABLETS safely and effectively. See full prescribing information for FAMOTIDINE TABLETS. Initial U.S. Approval: 1986 ...
-
Table of ContentsTable of Contents
-
1 Indications and UsageFamotidine tablets are indicated in adult and pediatric patients 40 kg and above for the treatment of: • active duodenal ulcer (DU). • active gastric ulcer(GU). • symptomatic non-erosive ...
-
2 Dosage and Administration2.1 Recommended Dosage - 2.1 Recommended Dosage - Table 1 shows the recommended dosage of Famotidine 20 mg and 40 mg tablets in adult and pediatric patients weighing 40 kg and greater with normal ...
-
3 Dosage Forms and Strengths• 20 mg tablets: round, white to off-white film-coated tablet coded CTI 121 on one side and the other side plain. • 40 mg tablets: round, white to off-white film-coated tablet coded CTI 122 ...
-
4 ContraindicationsFamotidine is contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis) to famotidine - or other histamine-2 (H - 2) receptor antagonists.
-
5 Warnings and PrecautionsWarnings and Precautions - 5.1 Central Nervous System Adverse Reactions - Central nervous system (CNS) adverse reactions, including confusion, delirium, hallucinations, disorientation, agitation ...
-
6 Adverse Reactions6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 Drug Interactions7.1 Drugs Dependent on Gastric pH for Absorption - Famotidine can reduce the absorption of other drugs, due to its effect on reducing intragastric acidity, leading to loss of efficacy of the ...
-
8 Use in Specific Populations8.1 Pregnancy - Risk Summary - Available data with H2-receptor antagonists, including famotidine, in pregnant women are insufficient to establish a drug-associated risk of major birth ...
-
10 OverdosageThe types of adverse reactions in overdosage of Famotidine are similar to the adverse reactions encountered with use of recommended dosages [see - Adverse Reactions ( 6.1)]. In the event ...
-
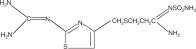
11 DescriptionThe active ingredient in Famotidine tablets is a histamine-2 (H2) receptor antagonist. Famotidine is N’-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide ...
-
12 Clinical Pharmacology12.1 Mechanism of Action - Famotidine is a competitive inhibitor of histamine-2 (H2) receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric ...
-
13 Nonclinical Toxicology13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic potential of famotidine was assessed in a 106-week oral ...
-
14 Clinical Studies14.1 Active Duodenal Ulcer - 14.1 Active Duodenal Ulcer - In a U.S. multicenter, double-blind trial in adult outpatients with endoscopically confirmed duodenal ulcer (DU), orally administered ...
-
17 Patient Counseling InformationCentral Nervous System (CNS) Adverse Reactions - Advise elderly patients and those with moderate and severe renal impairment of the risk of CNS adverse reactions, including confusion, delirium ...
-
16 How Supplied/Storage and HandlingFamotidine 40 mg tablets are white, round, film-coated tablets engraved with CTI 122 on one side, supplied as follows: NDC: 72162-1737-0: 1000 Tablets in a BOTTLE - NDC: 72162-1737-1: 100 Tablets ...
-
PRINCIPAL DISPLAY PANELFamotidine 40 mg Tablets
-
INGREDIENTS AND APPEARANCEProduct Information