Label: 50 PERSON ANSI- benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, water, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin, isopropyl alcohol kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 52124-0001-1, 52124-0002-1, 52124-0003-1, 52124-0004-1, view more52124-0005-1, 52124-0006-1, 52124-0008-1, 52124-0009-1, 52124-0010-1, 52124-0011-1, 52124-0113-1 - Packager: Genuine First Aid LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 7, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- WHEN USING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- WARNINGS
- DOSAGE & ADMINISTRATION
- PURPOSE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- DO NOT USE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: shock, facial swelling, asthma (wheezing) rash, skin reddening, blisters, hives If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach
bleeding. The chance is higher if you: are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug, take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen, or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor If:
you experience any of the following signs of stomach bleeding; feel faint; vomit blood; have bloody or black stools; have stomach pain that does get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; redness or swelling is present in the painful area; any new symptoms appear - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
do not use more than directed; the smallest effective dose should be used; do not take longer than 10 days, unless directed by a doctor.
Adults and Children (12 years and older): Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings:
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if: adult takes more than 12 tablets in 24 hours, which is the maximum daily amount; child takes more than 5 doses in 24 hours; taken with other drugs containing acetaminophen; adult has 3 or more alcoholic drinks every day while using this product - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Adults and Children Take 2 tablets every 4 to 6 hours as
12 years of age needed. Do not take more than 12 tablets
or older in 24 hours.
Children 6-11 years Take 1 tablet every 4 to 6 hours as
of age needed. Do not take more than 5
tablets in 24 hours.
Children under 6 Do not use this regular strength product.
years of age This will provide more than the
recommended dose (overdose) and could
cause serious health problems. - STORAGE AND HANDLING
- GENERAL PRECAUTIONS
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox of flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: hives, skin reddening, facial swelling, rash, asthma (wheezing), blisters, shock, If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
are age 60 or older; have had stomach ulcers or bleeding problems; take a blood thinner (anticoagulant) or steroid drug; take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others); have 3 or more alcoholic drinks every day while using this product; take more or for a longer time than directed - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
feel faint; vomit blood; have bloody or black stools; have stomach
pain that does not get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; you have difficulty swallowing; if ringing in the ears or loss of hearing occurs; redness or swelling is present in the painful areas; any new symptoms appear - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STORAGE AND HANDLING
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- ACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
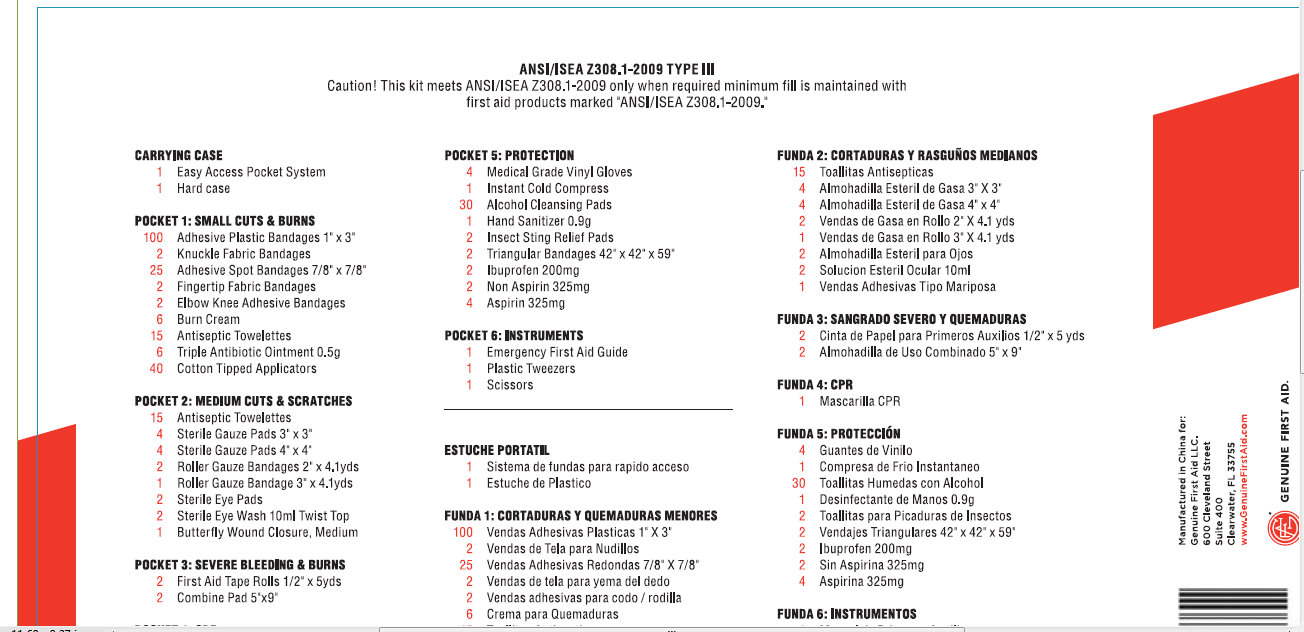
PRINCIPAL DISPLAY PANEL
ANSI/ISEA Z308.1-2009 TYPE III
Caution! This Kit meets ANSI/ISEA Z308.1-2009 only when required minimum fill
is maintained with first aid products marked "ANSI/ISEA Z308.1-2009."
CARRYING CASE
1 Easy Access Pocket System
1 Hard Case
POCKET 1: SMALL CUTS AND BURNS
100 Adhesive Plastic Bandages 1"x3"
2 Knuckle Fabric Bandage
25 Adhesive Spot Bandages 7/8"X7/8"
2 Fingertip
Fabric Bandage
2 Elbow Knee Adhesive Bandage
6 Burn Cream
15 Antiseptic
Towelettes
6 Triple Antibiotic Ointment 0.5gr
40 Cotton Tipped
Applicators
POCKET 2: MEDIUM CUTS AND SCRATCHES
15 Antiseptic
Towelettes
4 Sterile Gauze Pad 3"x3"
4 Sterile Gauze Pads 4"X4"
2
Roller Gauze Bandage 2"X4.1yds
1 Roller Gauze Bandage 3"X4.1yds
2 Sterile
Eye Pads
2 Sterile Eye Wash 10ml Twist Top
1 Butterfly Wound Closure,
Medium
POCKET 3: SEVERE BLEEDING AND BURNS
2 First Aid Tape Rolls 1/2"x5 yds.
2 Combine Pad 5"X9"
POCKET 4: CPR
1 CPR Breathing Barrier
POCKET 5: PROTECTION
4
Medical Grade Vinyl Gloves
1 Instant Cold Compress
30 Alcohol Cleansing
Pads
1 Hand Sanitizer 0.9g
2 Insect Sting Relief Pads
2 Triangular
Bandage 42"x42"x59"
2 Ibuprofen 200mg
2 Non Aspirin 325mg
4 Aspirin
325mg
POCKET 6: INSTRUMENTS
1 Emergency First Aid Guide
1 Plastic
Tweezers
1 Scissors
Manufactured in China for:
Genuine
First Aid LLC.
600 Cleveland Street
Suite 400
Clearwater FL
33755
www.GenuineFirstAid.com
GENUINE FIRST AID
Copyright c 2010 Genuine First Aid LLC. All rights reserved. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
50 PERSON ANSI
benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, water, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin, isopropyl alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52124-0113 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0113-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKAGE 5.4 g Part 2 30 PACKAGE 24 mL Part 3 6 TUBE 3 g Part 4 2 BOTTLE 20 mL Part 5 2 PACKAGE 1 mL Part 6 1 PACKAGE 2 Part 7 1 PACKAGE 2 Part 8 2 PACKAGE 4 Part 9 30 PACKAGE 15 mL Part 10 1 PACKAGE 0.9 g Part 1 of 10 GENUINE FIRST AID BURN ANTISEPTIC PAIN RELIEF WITH ALOE
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC:52124-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.5 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0004-1 0.9 g in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part345 06/02/2010 Part 2 of 10 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC:52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.40 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 06/02/2010 Part 3 of 10 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0003-1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 06/02/2010 Part 4 of 10 STERILE ISOTONIC BUFFERED GENUINE EYEWASH
water liquidProduct Information Item Code (Source) NDC:52124-0005 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.16 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0005-1 10 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 06/02/2010 Part 5 of 10 INSECT STING RELIEF PAD
benzocaine,alcohol liquidProduct Information Item Code (Source) NDC:52124-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mL in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0008-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 06/02/2010 Part 6 of 10 IBUPROFEN
ibuprofen tabletProduct Information Item Code (Source) NDC:52124-0009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (White) Score no score Shape ROUND Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0009-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075010 06/02/2010 Part 7 of 10 NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC:52124-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white (WHITE) Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0010-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 06/02/2010 Part 8 of 10 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC:52124-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (White) Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;157;ASPIRIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0011-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 06/02/2010 Part 9 of 10 ALCOHOL CLEANSING PAD
isopropyl alcohol liquidProduct Information Item Code (Source) NDC:52124-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0002-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 06/02/2010 Part 10 of 10 GENUINE HAND SANITIZER INSTANT ANTISEPTIC HANDWASH WITH VITAMIN E AND ALOE
alcohol gelProduct Information Item Code (Source) NDC:52124-0006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0006-1 0.9 g in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 06/02/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 06/02/2010 Labeler - Genuine First Aid LLC (619609857) Establishment Name Address ID/FEI Business Operations GFA Production ( Xiamen) Co., Ltd 421256261 manufacture