Label: ENVIO 90 DAY DAILY ESSENTIALS TRIO SUB SHIPMENT- benzoyl peroxide and salicylic acid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 81252-0102-1, 81252-0202-1, 81252-2001-1 - Packager: Smart Skin Health, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
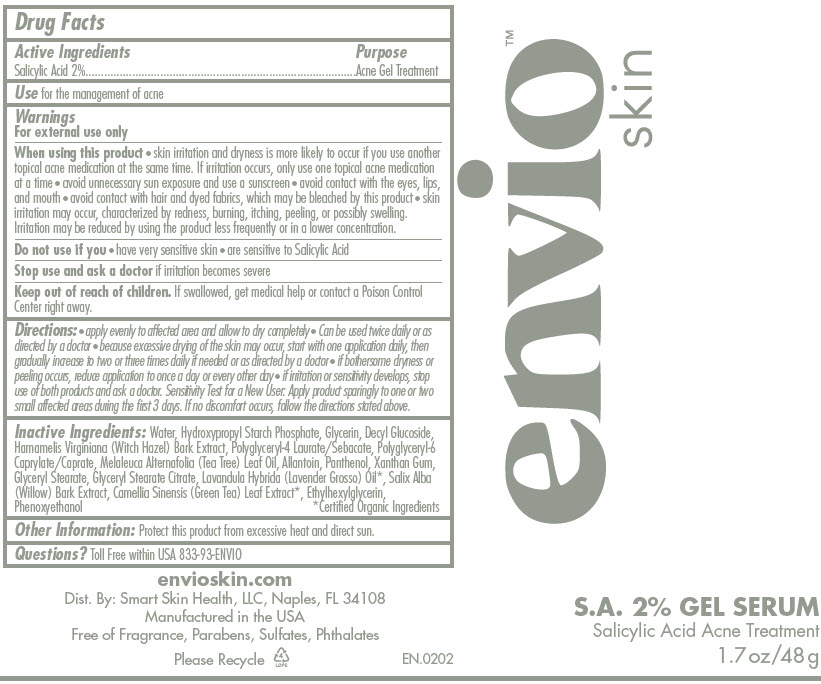
- Active Ingredients
- Purpose
- Use
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- apply a small amount to dampened skin
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product
- if irritation or sensitivity develops, stop use of both products and ask a doctor. Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
- Inactive Ingredients
- Other Information
- Questions or Comments
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Use
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- apply evenly to affected area and allow to dry completely
- Can be used twice daily or as directed by a doctor
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if irritation or sensitivity develops, stop use of both products and ask a doctor. Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
-
Inactive Ingredients
Water, Hydroxypropyl Starch Phosphate, Glycerin, Decyl Glucoside, Hamamelis Virginiana (Witch Hazel) Bark Extract, Polyglyceryl-4 Laurate/Sebacate, Polyglyceryl-6 Caprylate/Caprate, Melaleuca Alternafolia (Tea Tree) Leaf Oil, Allantoin, Panthenol, Xanthan Gum, Glyceryl Stearate, Glyceryl Stearate Citrate, Lavandula Hybrida (Lavender Grosso) Oil1, Salix Alba (Willow) Bark Extract, Camellia Sinensis (Green Tea) Leaf Extract1, Ethylhexylglycerin, Phenoxyethanol
- 1
- Certified Organic Ingredients
- Other Information
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
- PRINCIPAL DISPLAY PANEL - 133 mL Tube Label
- PRINCIPAL DISPLAY PANEL - 48 g Tube Label
-
INGREDIENTS AND APPEARANCE
ENVIO 90 DAY DAILY ESSENTIALS TRIO SUB SHIPMENT
benzoyl peroxide and salicylic acid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81252-2001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81252-2001-1 1 in 1 CARTON 10/21/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 133 mL Part 2 1 TUBE 48 g Part 3 1 TUBE 45 mL Part 1 of 3 B.P.O. CLEANSER
benzoyl peroxide gelProduct Information Item Code (Source) NDC:81252-0102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzoyl Peroxide (UNII: W9WZN9A0GM) (Benzoyl Peroxide - UNII:W9WZN9A0GM) Benzoyl Peroxide 5 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sodium C14-16 Olefin Sulfonate (UNII: O9W3D3YF5U) Glycerin (UNII: PDC6A3C0OX) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) Cetostearyl Alcohol (UNII: 2DMT128M1S) Glycol Distearate (UNII: 13W7MDN21W) Cetyl Alcohol (UNII: 936JST6JCN) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Hyaluronate Sodium (UNII: YSE9PPT4TH) Allantoin (UNII: 344S277G0Z) Ethylhexylglycerin (UNII: 147D247K3P) Phenoxyethanol (UNII: HIE492ZZ3T) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81252-0102-1 133 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/21/2021 Part 2 of 3 S.A. SERUM
salicylic acid gelProduct Information Item Code (Source) NDC:81252-0202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 2 g in 100 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Decyl Glucoside (UNII: Z17H97EA6Y) Witch Hazel (UNII: 101I4J0U34) Tea Tree Oil (UNII: VIF565UC2G) Allantoin (UNII: 344S277G0Z) Panthenol (UNII: WV9CM0O67Z) Xanthan Gum (UNII: TTV12P4NEE) Glyceryl Monostearate (UNII: 230OU9XXE4) Glyceryl Stearate Citrate (UNII: WH8T92A065) Ylang-Ylang Oil (UNII: 8YOY78GNNX) Salix Alba Bark (UNII: 205MXS71H7) Green Tea Leaf (UNII: W2ZU1RY8B0) Ethylhexylglycerin (UNII: 147D247K3P) Phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81252-0202-1 48 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/21/2021 Part 3 of 3 HYDRATOR
moisturizing gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR Glycerin (UNII: PDC6A3C0OX) INGR Totarol (UNII: 67NH2854WW) INGR Aloe Vera Leaf (UNII: ZY81Z83H0X) INGR Ylang-Ylang Oil (UNII: 8YOY78GNNX) INGR Sandalwood (UNII: 3641YW25N2) INGR Lavender Oil (UNII: ZBP1YXW0H8) INGR Camphor Oil (UNII: 75IZZ8Y727) INGR Orange Oil, Cold Pressed (UNII: AKN3KSD11B) INGR Rosa X Damascena Flower Oil (UNII: 18920M3T13) INGR Lonicera Japonica Flower (UNII: 4465L2WS4Y) INGR Lonicera Caprifolium Flower (UNII: 5N1WD9784U) INGR Chlorophyll (UNII: 00WNZ48OR9) INGR Hamamelis Virginiana Top Water (UNII: NT00Y05A2V) INGR Alcohol (UNII: 3K9958V90M) INGR Citric Acid Monohydrate (UNII: 2968PHW8QP) INGR Maltodextrin (UNII: 7CVR7L4A2D) INGR Trisodium Ethylenediamine Disuccinate (UNII: YA22H34H9Q) INGR Carbomer Homopolymer, Unspecified Type (UNII: 0A5MM307FC) INGR Sodium Hydroxide (UNII: 55X04QC32I) INGR Sodium Metabisulfite (UNII: 4VON5FNS3C) INGR Acetyltributyl Citrate (UNII: 0ZBX0N59RZ) INGR Phenylethyl Alcohol (UNII: ML9LGA7468) INGR Caprylyl Glycol (UNII: 00YIU5438U) INGR Potassium Sorbate (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 45 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/21/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/21/2021 Labeler - Smart Skin Health, LLC (117039871)