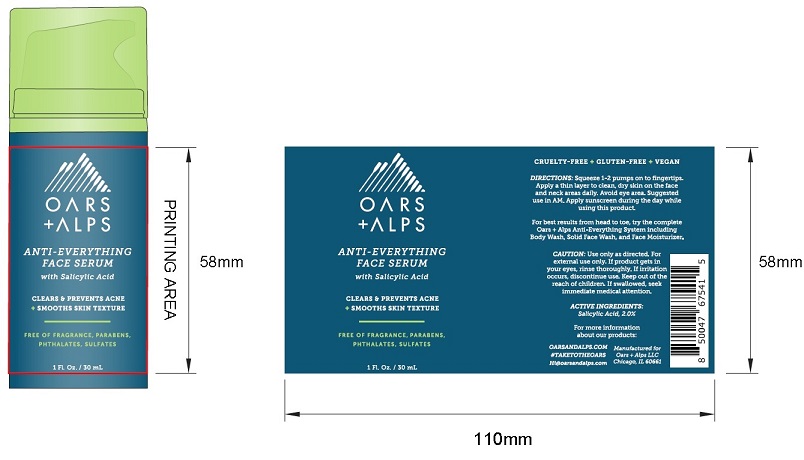
Label: ANTI-EVERYTHING FACE SERUM- salicylic acid serum liquid
- NDC Code(s): 54111-180-01
- Packager: Bentley laboratories, llC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use onlyDo not use if you have very sensitive
skin or known allergy to aspirinSlop use and ask a doctor if
excessive itching, dryness, redness,
burning, or swelling occurs or if these
symptoms persistWhen ullng this product • Keep
away from eyes, lips, and other
mucous membranes. If contact occurs,
flush thoroughly with water.• Using
other topical acne medications at the
same time or immediately following
use of this product may increase
dryness or irritation of the skin. If this
occurs, only one medication should be
used unless directed by doctor. - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
• Squeeze 1-2 pumps on to fingertlps.
Apply a thin layer to clean,
dry skin in the morning. Avoid eye
area. Suggested use In AM.• At first, use every other day. Then
up to twice a day as tolerated
• Use sunscreen during the day while
using this product
• Because excessive drying of the skin
may occur, start with one application
daily, then gradually increase if needed
or as directed by a doctor.
• If bothersome dryness or peeling
occurs, reduce the application.
• Store dry at room temperature -
INACTIVE INGREDIENT
Inactive ingredients Water,
Glycolic Acid, Isopentyldiol, Glycerin,
HDI/Trimethylol Hexyllactone
Crosspolymer, Cetearyl Alcohol,
Ammonium Acryloyldimethyltaurate/VP
Copolymer, Sodium Hydroxide, Tapioca
Starch, C13-15 Alkane, Phenoxyethanol,
1,2-Hexanediol, Panthenol, Eucalyptus
Globulus Leaf Oil, Tetrasodium Glutamate
Diacetate, Cladonia Stellaris Extract,
Silica, Sodium Hyaluronate, Iron Oxides
(CI 77492), Ricinus Communis (Castor)
Seed Oil, Niacinamide,
Chlorophyllin-Copper Complex, Limonene - SPL UNCLASSIFIED SECTION
- QUESTIONS
- Product Packaging: OARS + ALPS ANTI-EVERYTHING FACE SERUM
-
INGREDIENTS AND APPEARANCE
ANTI-EVERYTHING FACE SERUM
salicylic acid serum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54111-180 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) Salicylic Acid 2.0 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCOLIC ACID (UNII: 0WT12SX38S) ISOPENTYLDIOL (UNII: 19NOL5474Q) GLYCERIN (UNII: PDC6A3C0OX) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) SODIUM HYDROXIDE (UNII: 55X04QC32I) STARCH, TAPIOCA (UNII: 24SC3U704I) C13-15 ALKANE (UNII: 114P5I43UJ) PHENOXYETHANOL (UNII: HIE492ZZ3T) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PANTHENOL (UNII: WV9CM0O67Z) EUCALYPTUS OIL (UNII: 2R04ONI662) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) CLADONIA CRISTATELLA WHOLE (UNII: YWE2XUB3DS) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CASTOR OIL (UNII: D5340Y2I9G) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM COPPER CHLOROPHYLLIN (UNII: 1D276TYV9O) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54111-180-01 1 in 1 CARTON 08/08/2024 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/08/2024 Labeler - Bentley laboratories, llC (068351753)