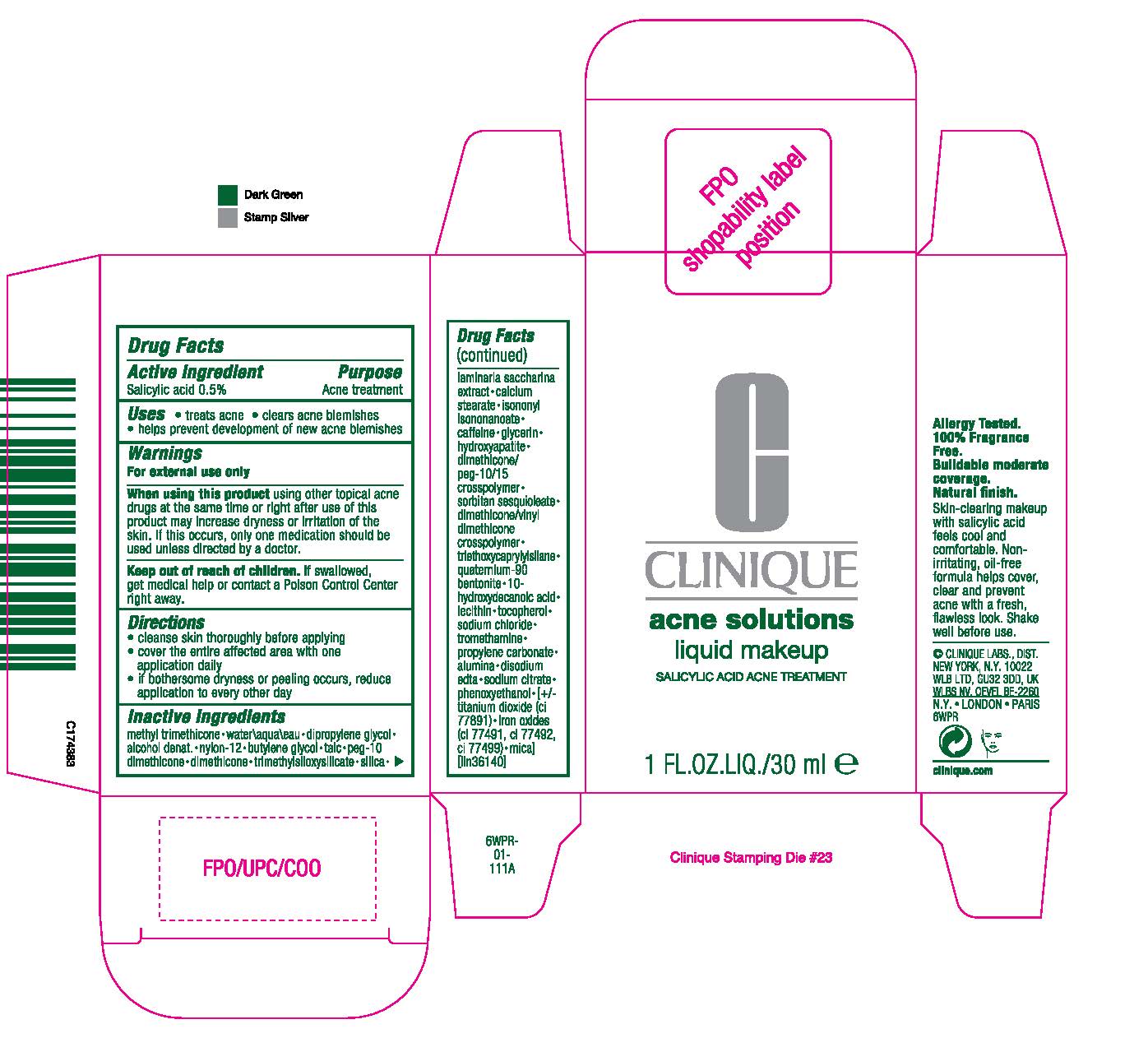
Label: ACNE SOLUTIONS LIQUID MAKEUP SALICYLIC ACID ACNE TREATMENT- salicylic acid liquid
- NDC Code(s): 49527-751-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:
METHYL TRIMETHICONE [] WATER [] DIPROPYLENE GLYCOL [] ALCOHOL DENAT. [] NYLON-12 [] BUTYLENE GLYCOL [] TALC [] PEG-10 DIMETHICONE [] DIMETHICONE [] TRIMETHYLSILOXYSILICATE [] SILICA [] LAMINARIA SACCHARINA EXTRACT [] CALCIUM STEARATE [] ISONONYL ISONONANOATE [] CAFFEINE [] GLYCERIN [] HYDROXYAPATITE [] DIMETHICONE/PEG-10/15 CROSSPOLYMER [] SORBITAN SESQUIOLEATE [] DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER [] TRIETHOXYCAPRYLYLSILANE [] QUATERNIUM-90 BENTONITE [] 10-HYDROXYDECANOIC ACID [] LECITHIN [] TOCOPHEROL [] SODIUM CHLORIDE [] TROMETHAMINE [] PROPYLENE CARBONATE [] ALUMINA [] DISODIUM EDTA [] SODIUM CITRATE [] PHENOXYETHANOL MAY CONTAIN: TITANIUM DIOXIDE [] IRON OXIDES[] MICA ILN36140
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE SOLUTIONS LIQUID MAKEUP SALICYLIC ACID ACNE TREATMENT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-751 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE CARBONATE (UNII: 8D08K3S51E) ALUMINUM OXIDE (UNII: LMI26O6933) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) WATER (UNII: 059QF0KO0R) DIPROPYLENE GLYCOL (UNII: E107L85C40) ALCOHOL (UNII: 3K9958V90M) NYLON-12 (UNII: 446U8J075B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TALC (UNII: 7SEV7J4R1U) BIS-PEG-10 DIMETHICONE/DIMER DILINOLEATE COPOLYMER (UNII: CF5W1YCX11) DIMETHICONE (UNII: 92RU3N3Y1O) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) CALCIUM STEARATE (UNII: 776XM7047L) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CAFFEINE (UNII: 3G6A5W338E) GLYCERIN (UNII: PDC6A3C0OX) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) 10-HYDROXYDECANOIC ACID (UNII: NP03XO416B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CHLORIDE (UNII: 451W47IQ8X) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-751-01 1 in 1 CARTON 12/04/2020 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/04/2020 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations PALC 078364654 pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(49527-751) , pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations Northtec LLC 943871157 pack(49527-751) , label(49527-751)