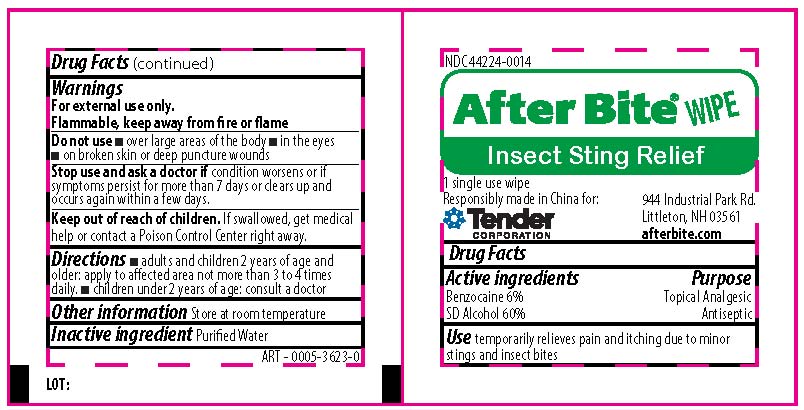
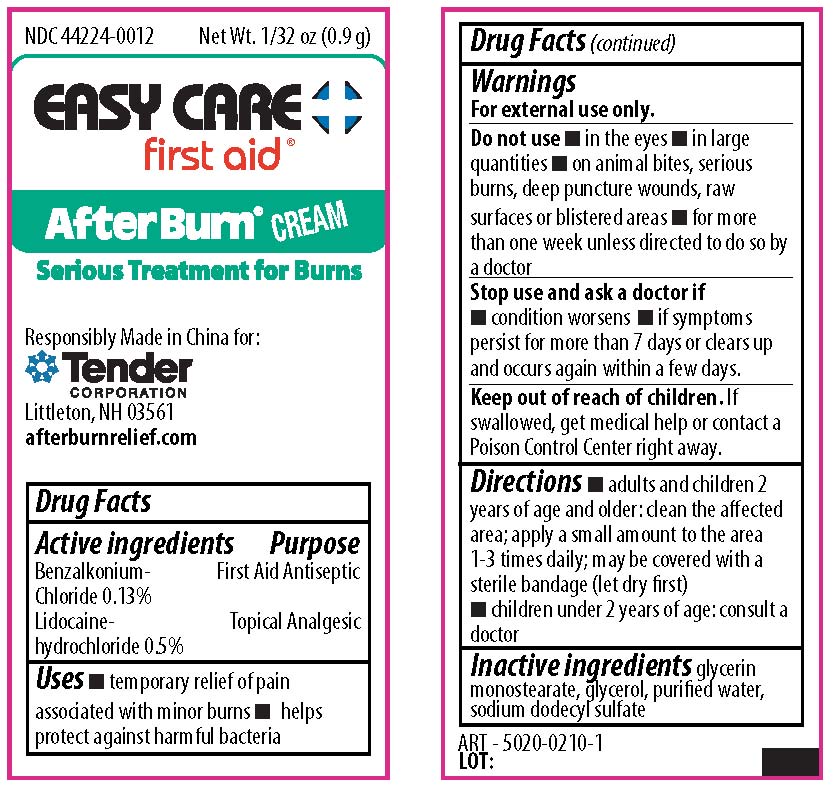
Label: CVS HEALTH ALL PURPOSE KIT- isopropyl alcohol, bacitracin zinc,neomycin sulfate,polymyxin b sulfate, benzocaine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 44224-0010-0, 44224-0012-1, 44224-0013-4, 44224-0014-1, view more69842-419-01 - Packager: CVS Pharmacy Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredient
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- DO NOT USE
- Ask a doctor before use if you have
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive Ingredients
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- DO NOT USE
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENT
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- DO NOT USE
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS HEALTH ALL PURPOSE KIT
isopropyl alcohol, bacitracin zinc,neomycin sulfate,polymyxin b sulfate, benzocaine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-419 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-419-01 1 in 1 KIT; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 09/01/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 12 POUCH 6 mL Part 2 3 TUBE 2.7 g Part 3 3 PACKAGE 3000 mg Part 4 2 PACKET 1.8 g Part 1 of 4 EASY CARE FIRST AID SKIN CLEANSING ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC:44224-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44224-0010-0 0.5 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/02/2019 Part 2 of 4 EASY CARE FIRST AID TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:44224-0013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44224-0013-4 0.9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/01/2019 Part 3 of 4 AFTER BITE WIPE INSECT STING RELIEF
benzocaine,alcohol swabProduct Information Item Code (Source) NDC:44224-0014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mg in 100 mg ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44224-0014-1 1000 mg in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 09/01/2019 Part 4 of 4 EASY CARE FIRST AID AFTERBURN
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC:44224-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.5 g in 100 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44224-0012-1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2019 Labeler - CVS Pharmacy Inc (062312574) Establishment Name Address ID/FEI Business Operations Tender Corporation 064437304 manufacture(69842-419)