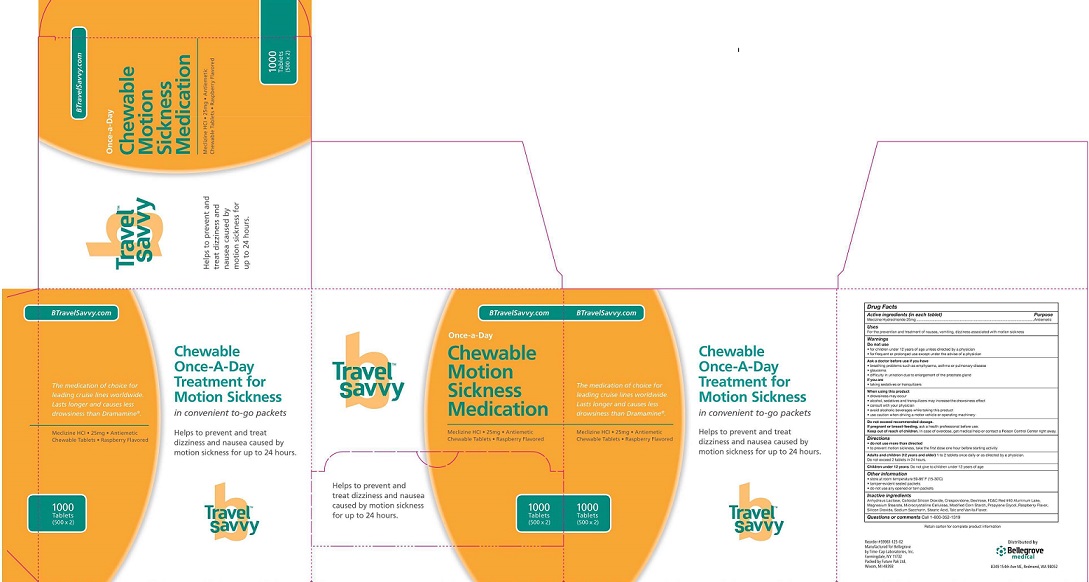
Label: MECLINZINE HYDORCHLORIDE- meclinzine hydrochloride - chewable tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 59961-125-01, 59961-125-02 - Packager: Bellegrove Medical Supply, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNINGS
Do not use
• for children under 12 years of age unless unless directed by a physician
• for frequent or prolonged use except under the advice of a physicianAsk a doctor before use if you have
breathing problems such as emphysema, asthma or pulmonary disease
glaucomaif you are
- taking sedatives or tranquilizers
When using this product
drowsiness may occur
alcohol, sedatives and tranquilizers may increase the drowsiness effect
consult with your physician
avoid alcoholic beverages while taking this product
use caution when driving a motor vehicle or operating machineryDo not exceed recommended dosage
If preganant or breast-feeding, ask a health professional before use
Keep out of reach of children. In case of overdose, get medical help or contact Poison Control Center right away
Other information
- store at room temperature 59-86 degrees F (15-30 degreesC)
- tamper-evident sealed packets
- do not use any opened or torn packets
-
DOSAGE AND ADMINISTAATION
Directions
do not use more than directed
to provent motion sickness, take the first dose one hour before starting activity
Adults and children (12 years and older)1 to 2 tablets once daily or as directed by a Physician
Do not exceed 2 tablets in 24 hours
Children under 12 years of age: Do not give to children under 12 years of age
- INDICATION AND USAGE
- OTC-KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- Active ingredients
- Inactive ingredients
- Package label
-
INGREDIENTS AND APPEARANCE
MECLINZINE HYDORCHLORIDE
meclinzine hydrochloride - chewable tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59961-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Meclizine hydrochloride (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) Meclizine hydrochloride 25 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) 1 mg Product Characteristics Color pink Score no score Shape ROUND Size 10mm Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59961-125-01 2 in 1 PACKAGE 01/01/2012 1 NDC:59961-125-02 500 in 1 BOX; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part336 01/01/2012 Labeler - Bellegrove Medical Supply, Inc. (141647094) Registrant - Bellegrove Medical Supply, Inc. (141647094) Establishment Name Address ID/FEI Business Operations Future Pak, Ltd. 087737672 api manufacture(59961-125) , pack(59961-125) , relabel(59961-125)