Label: ALCOHOL-FREE WIPES cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 79805-011-01 - Packager: JUXIN (Jiangsu) New Material Packaging Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Other information
- Do not flush.
- Rinse toys or food with clean water after cleaning.
- Do not use as a diaper wipe or on sensitive skin.
- Do not use on unpainted wood.
- Do not use to clean dishes, glassware or utensils.
- Store in original container in areas inaccessible to children.
- Keep securely closed.
- Do not reuse or refill this container.
- Discard empty container in trash or recycle.
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALCOHOL-FREE WIPES
alcohol-free wipes clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79805-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 mg in 100 mL Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) BRONOPOL (UNII: 6PU1E16C9W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79805-011-01 15 in 1 PACKET 08/20/2020 1 3.65 mL in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 08/20/2020 Labeler - JUXIN (Jiangsu) New Material Packaging Co., Ltd. (546994282) Establishment Name Address ID/FEI Business Operations JUXIN (Jiangsu) New Material Packaging Co., Ltd. 546994282 manufacture(79805-011)


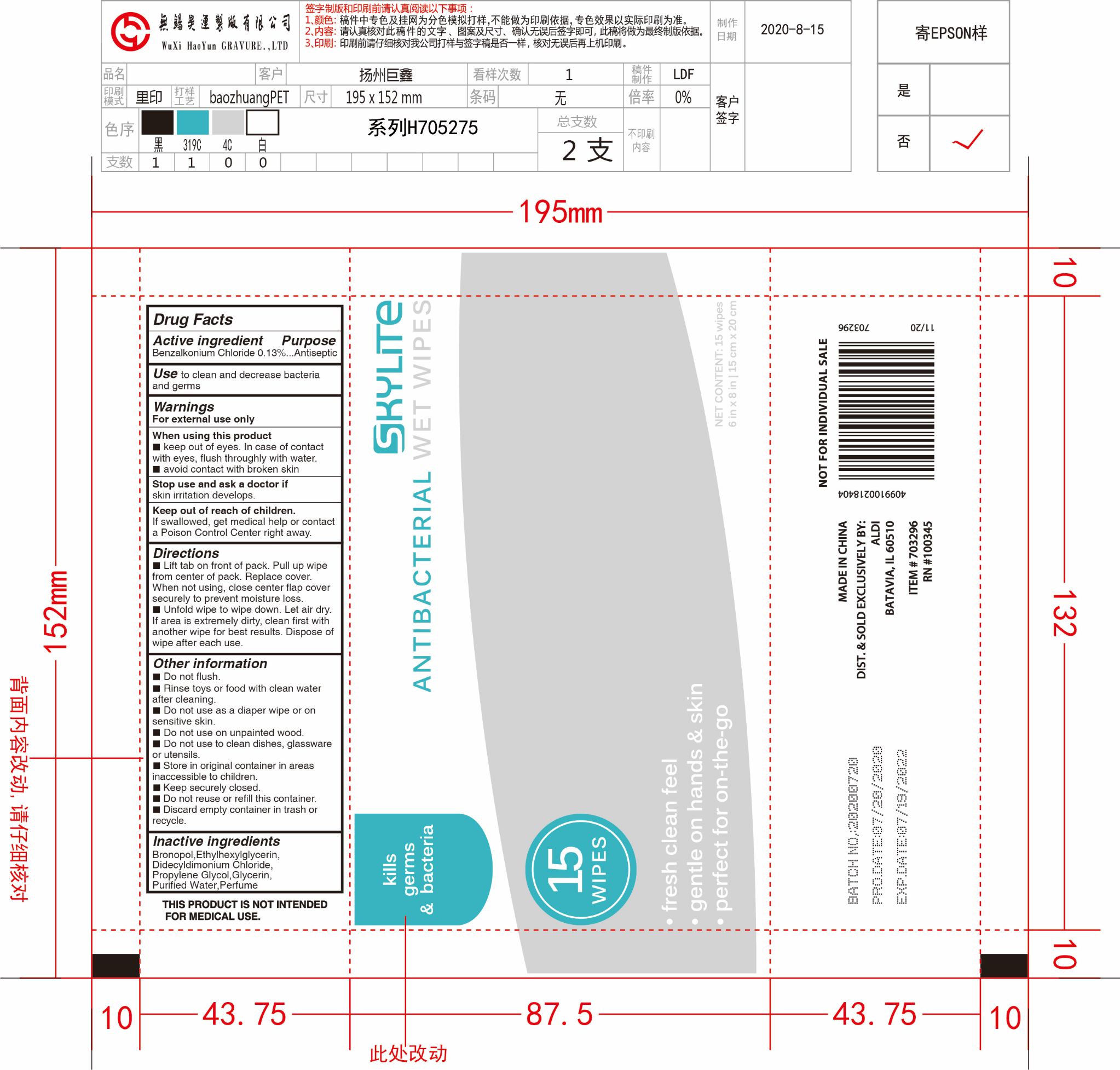 15pcs NDC: 79805-011-01
15pcs NDC: 79805-011-01