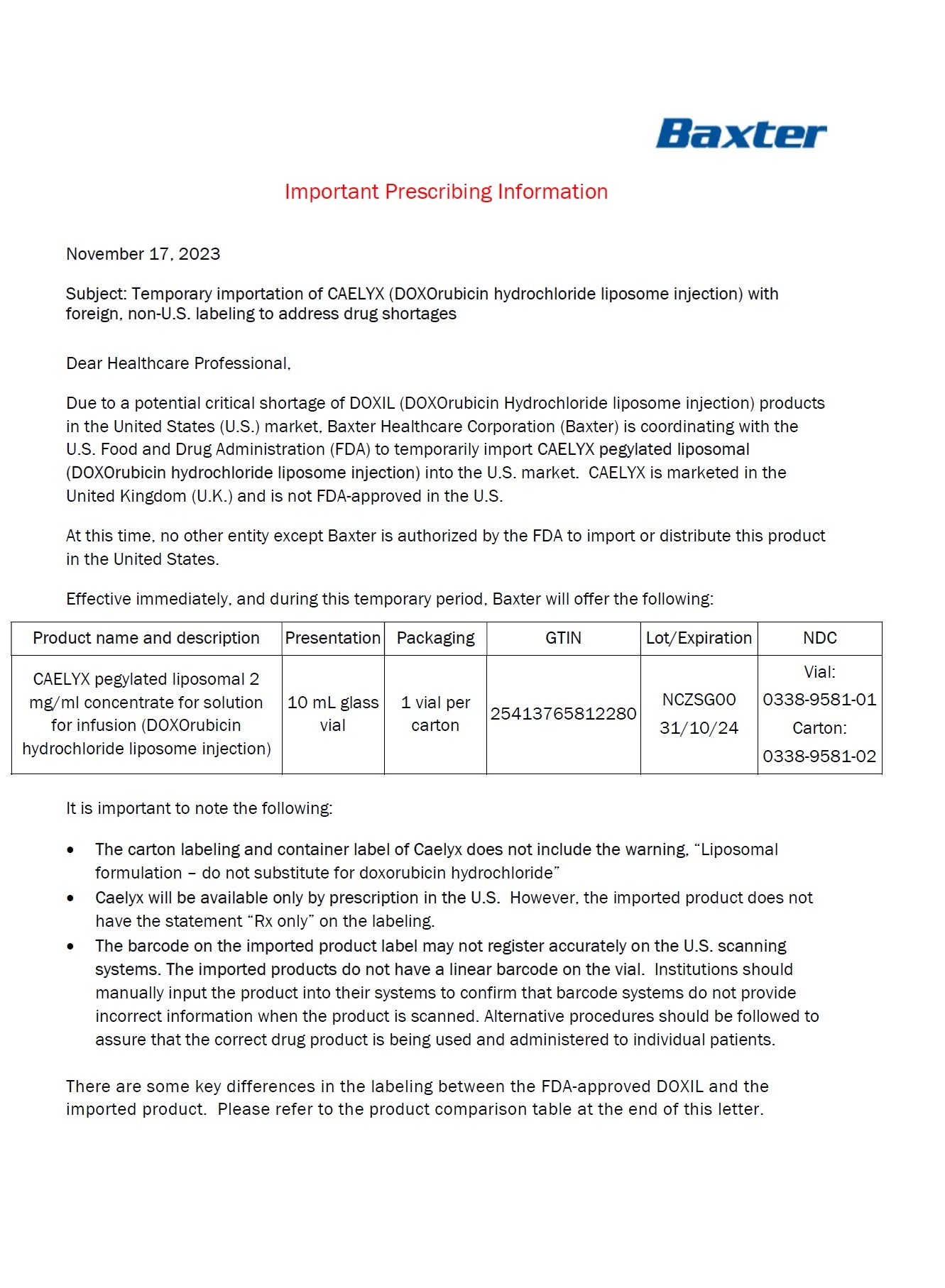
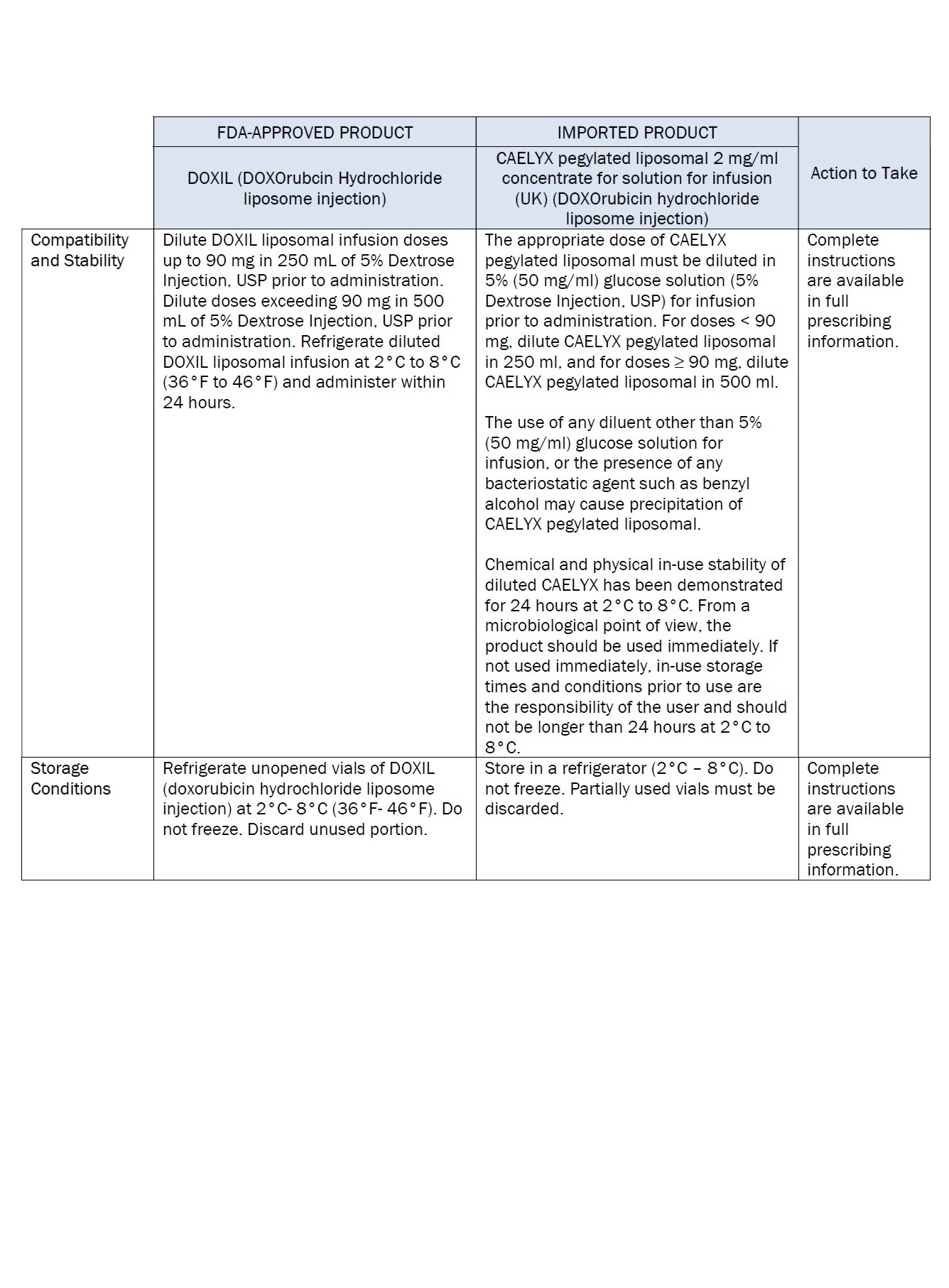
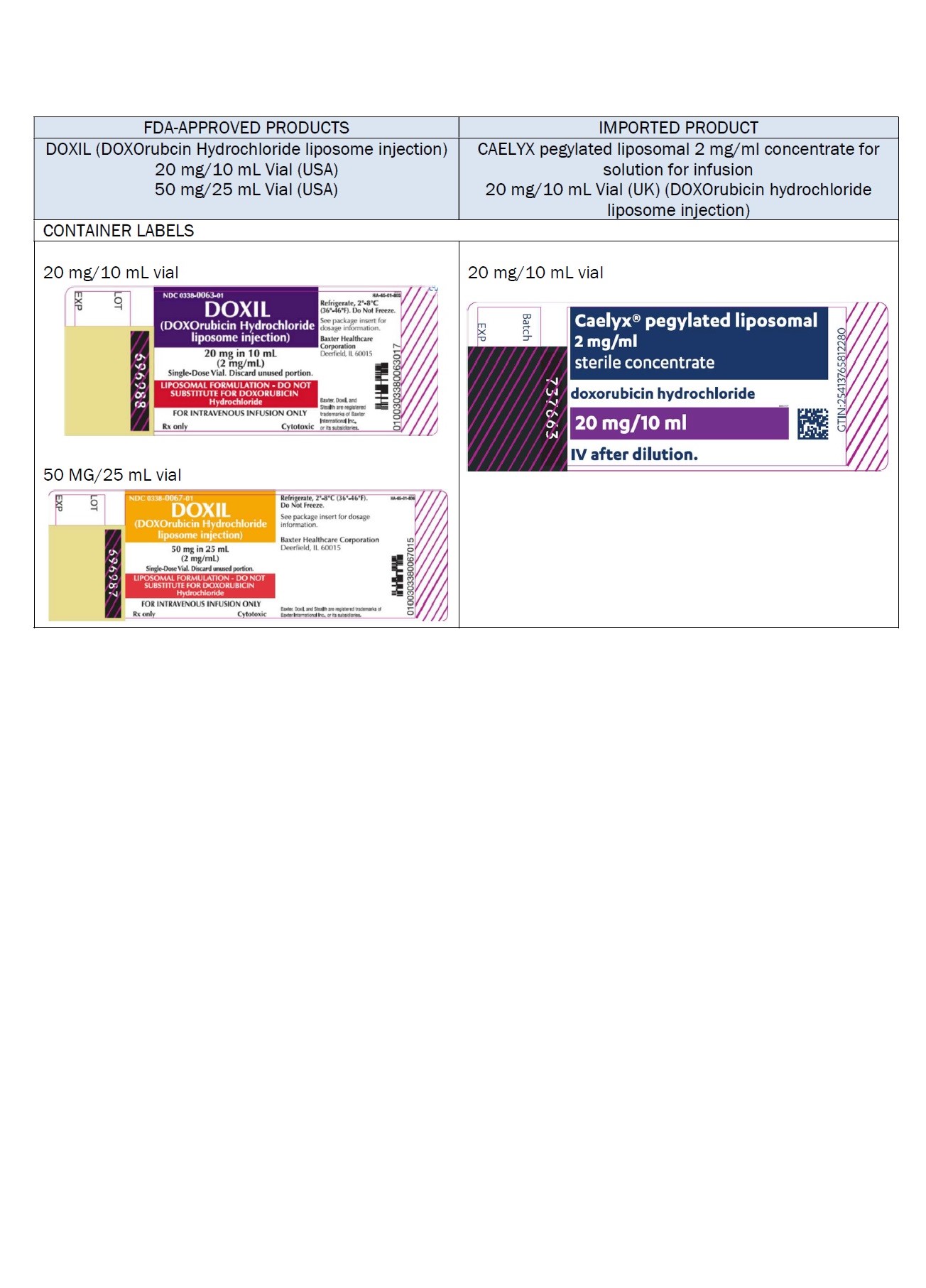
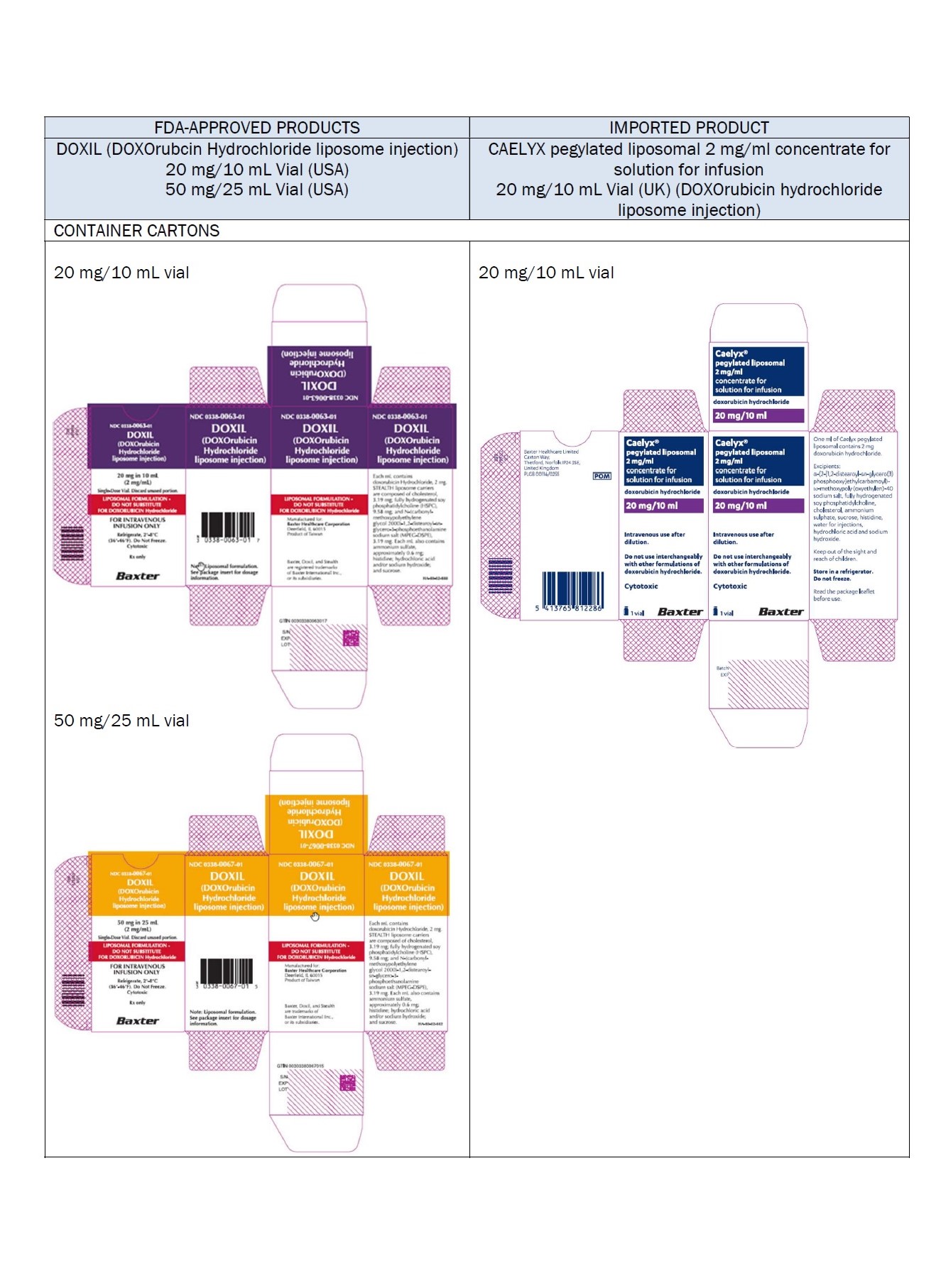
Label: DOXORUBICIN HYDROCHLORIDE, LIPOSOMAL- doxorubicin hydrochloride injection, suspension, liposomal
- NDC Code(s): 0338-9581-01, 0338-9581-02
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HEALTH CARE PROVIDER LETTER
-
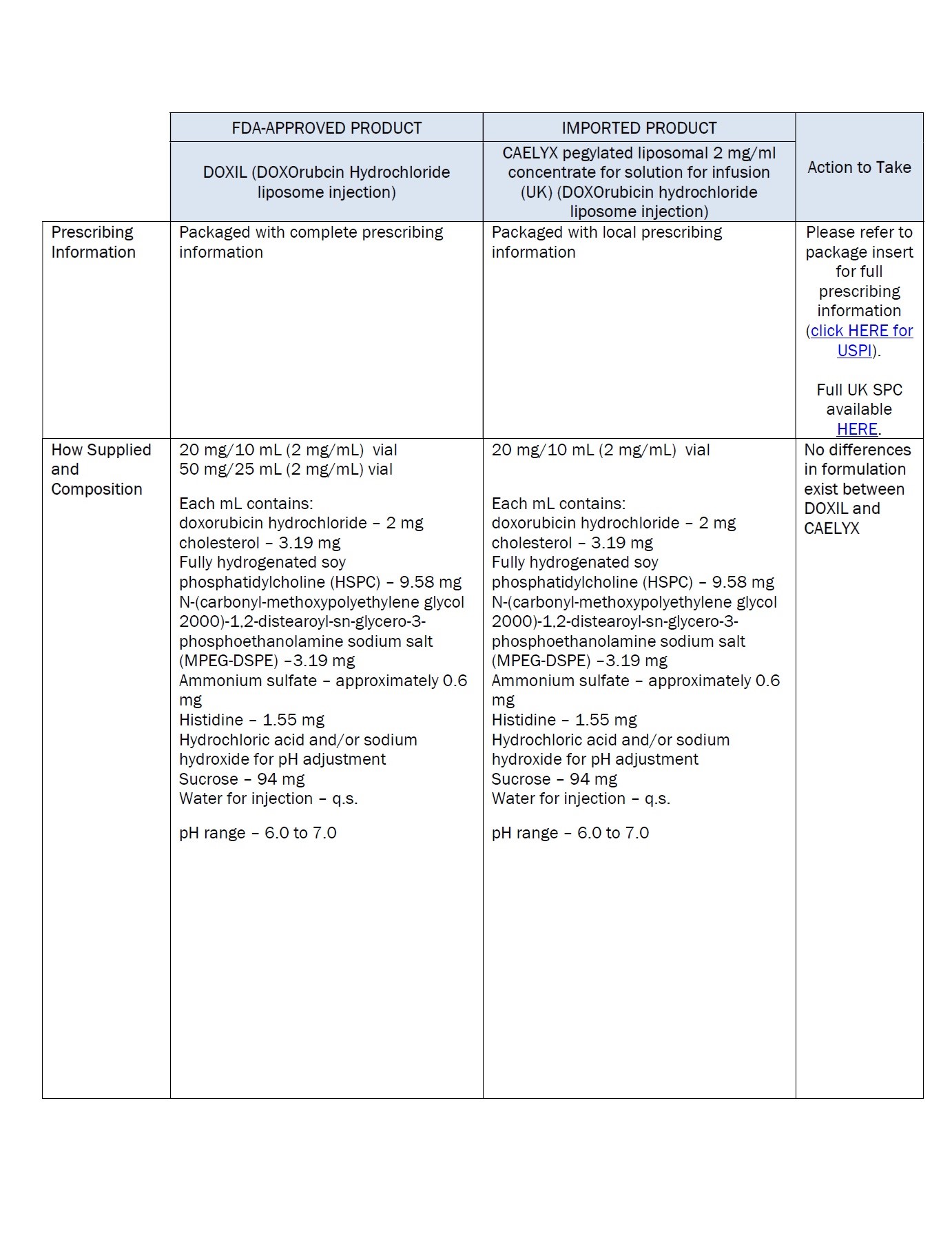
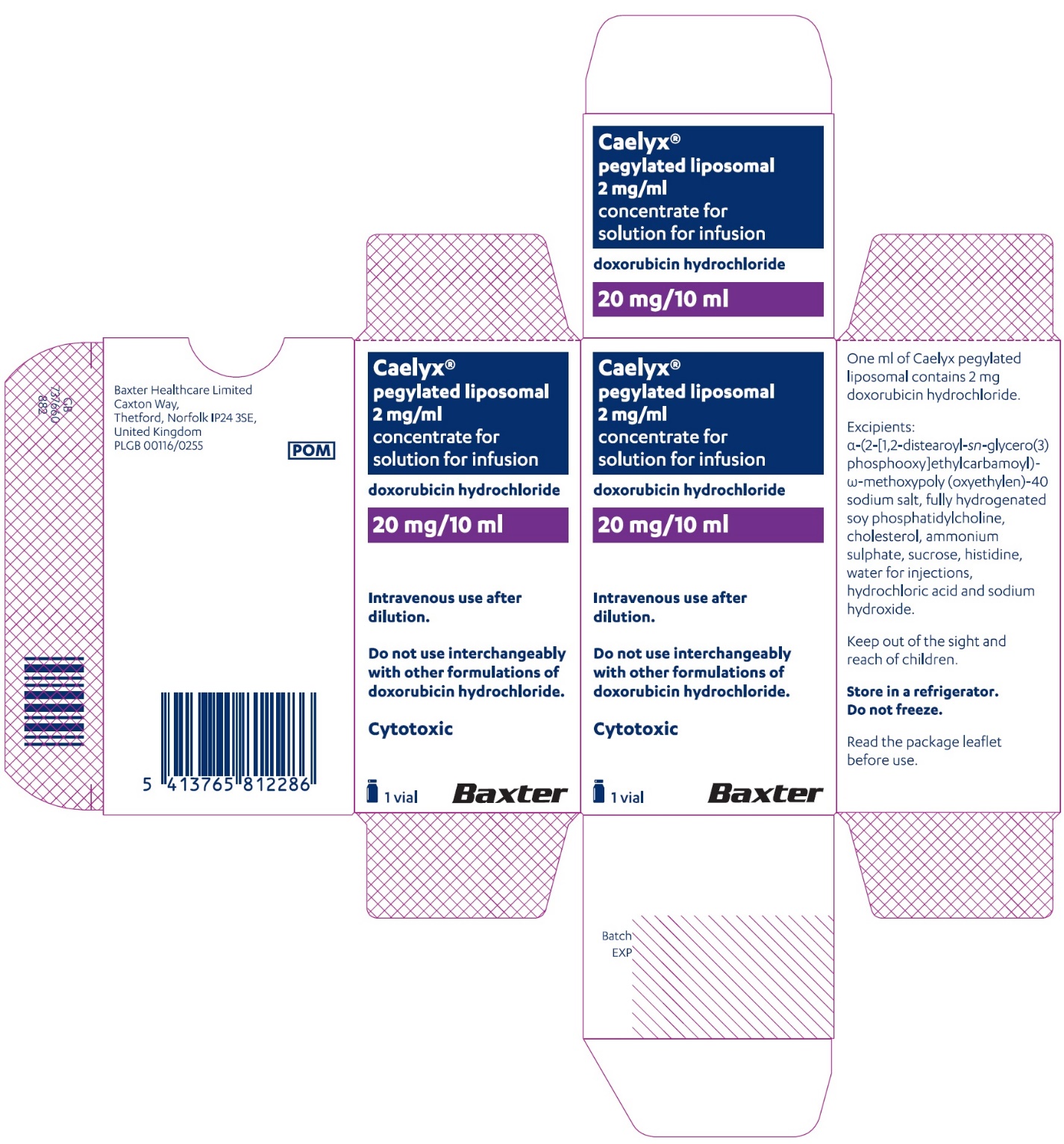
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 20 mg/10 mL
Caelyx® pegylated liposomal
2 mg/ml
sterile concentrate
doxorubicin hydrochloride
20 mg/10 mL
IV after dilution.
Caelyx®
pegylated liposomal
2 mg/ml
concentrate for
solution for infusion
doxorubicin hydrochloride
20 mg/10 mL
Intravenous use after
dilution.
Do not use interchangeably
with other formulations of
doxorubicin hydrochloride.Cytotoxic
1 vial
Baxter Logo
One ml of Caelyx pegylated
liposomal contains 2 mg
doxorubicin hydrochloride.Excipients: α-(2-[1,2-distearoyl-sn-glycero(3)
phosphooxy]ethylcarbamoyl)
-ϖ-methoxypoly(oxyethylen)-40
sodium salt, fully hydrogenated
soy phosphatidylcholine,
cholesterol, ammonium
sulphate, sucrose, histidine,
water for injections,
hydrochloric acid and sodium
hydroxide.Keep out of the sight and
reach of children.Store in a refrigerator.
Do not freeze.Read the package leaflet
before use.Baxter Healthcare Limited
Caxton Way,
Thetford,
Norfolk,
IP24 3SE,
United Kingdom
PLGB 00116/0255
-
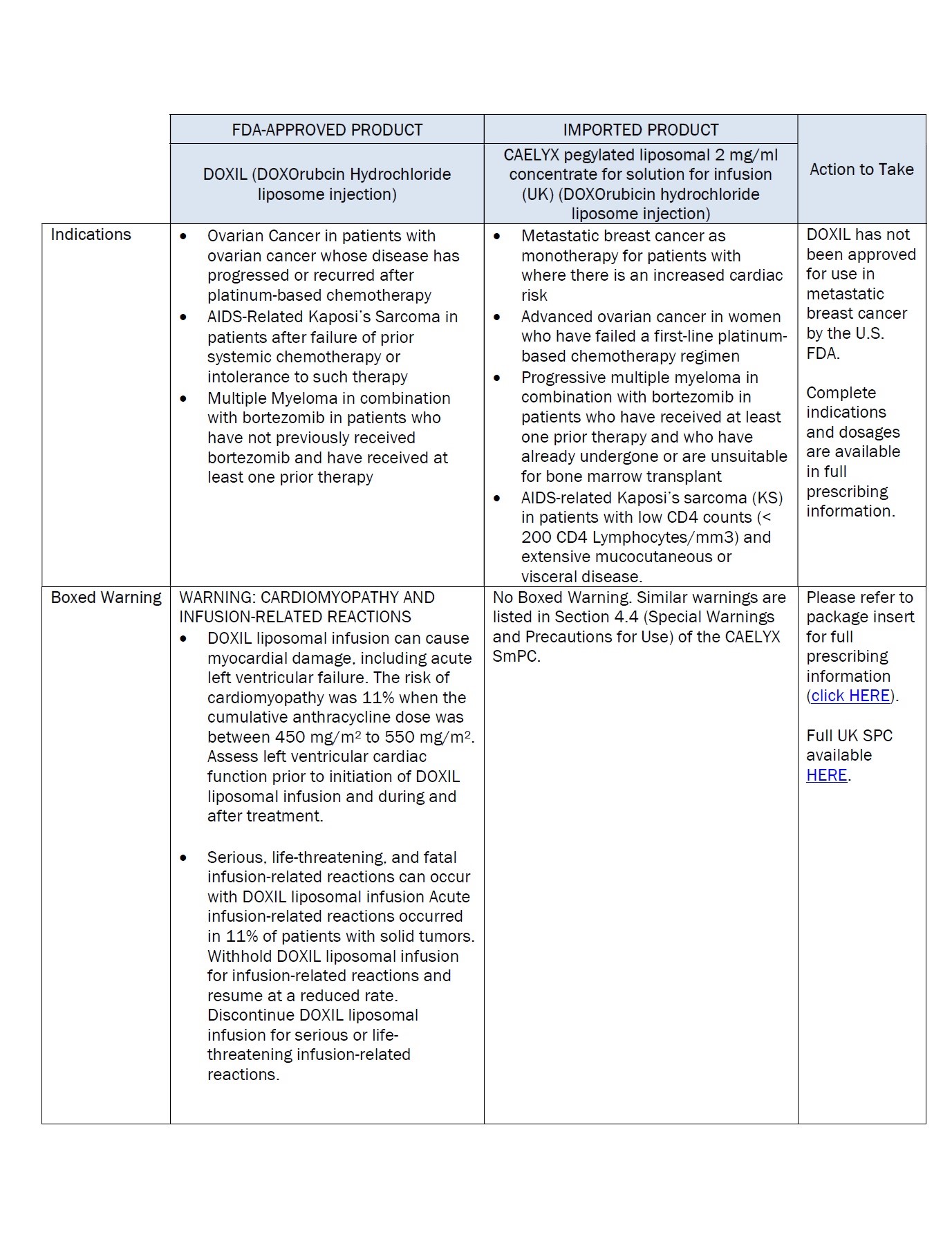
INGREDIENTS AND APPEARANCE
DOXORUBICIN HYDROCHLORIDE, LIPOSOMAL
doxorubicin hydrochloride injection, suspension, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9581 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength doxorubicin hydrochloride (UNII: 82F2G7BL4E) (doxorubicin - UNII:80168379AG) doxorubicin hydrochloride 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM N-(CARBONYL-METHOXYPOLYETHYLENE GLYCOL 2000)-1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOETHANOLAMINE (UNII: 3L6NN8ZZKU) 3.19 mg in 1 mL hydrogenated soybean lecithin (UNII: H1109Z9J4N) 9.58 mg in 1 mL cholesterol (UNII: 97C5T2UQ7J) 3.19 mg in 1 mL ammonium sulfate (UNII: SU46BAM238) 0.6 mg in 1 mL histidine (UNII: 4QD397987E) 1.55 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) sucrose (UNII: C151H8M554) 94 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9581-02 1 in 1 CARTON 12/19/2023 1 NDC:0338-9581-01 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 12/19/2023 Labeler - Baxter Healthcare Corporation (005083209)