Label: CLINDAMYCIN PHOSPHATE gel
- NDC Code(s): 73473-300-30, 73473-300-60
- Packager: Solaris Pharma Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Clindamycin phosphate gel USP, 1% contains clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per gram.
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin.
The gel contains allantoin, carbomer 974P, methylparaben, polyethylene glycol 400, propylene glycol, sodium hydroxide, and purified water.
The structural formula is represented below:
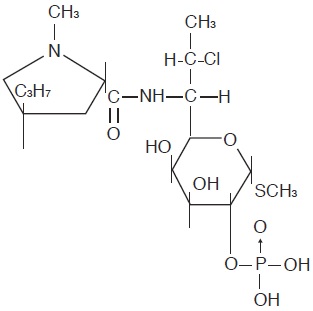
The chemical name for clindamycin phosphate is Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate). -
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of clindamycin in treating acne vulgaris is unknown.
Pharmacokinetics
Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin.
Microbiology
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is bacteriostatic.
Antimicrobial Activity
Clindamycin is active in vitro against most isolates of Propionibacterium acnes; however, the clinical significance is unknown.
Resistance
Resistance to clindamycin is most often caused by modification of specific bases of the 23S ribosomal RNA. Cross-resistance between clindamycin and lincomycin is complete. Because the binding sites for these antibacterial drugs overlap, cross resistance is sometimes observed among lincosamides, macrolides and streptogramin B. Macrolide-inducible resistance to clindamycin occurs in some isolates of macrolide-resistant bacteria.
-
INDICATIONS AND USAGE
Clindamycin phosphate gel, 1% is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should consider whether other agents are more appropriate (see CONTRAINDICATIONS, WARNINGS and ADVERSE REACTIONS).
- CONTRAINDICATIONS
-
WARNINGS
Orally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of the antibiotic from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin.
Studies indicate a toxin(s) produced by clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Endoscopic examination may reveal pseudomembranous colitis. Stool culture for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
When significant diarrhea occurs, the drug should be discontinued. Large bowel endoscopy should be considered to establish a definitive diagnosis in cases of severe diarrhea.
Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen the condition. Vancomycin has been found to be effective in the treatment of antibiotic-associated pseudomembranous colitis produced by Clostridium difficile. The usual adult dosage is 500 milligrams to 2 grams of vancomycin orally per day in three to four divided doses administered for 7 to 10 days. Cholestyramine or colestipol resins bind vancomycin in vitro. If both a resin and vancomycin are to be administered concurrently, it may be advisable to separate the time of administration of each drug.
Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of oral and parenteral therapy with clindamycin.
-
PRECAUTIONS
General
Clindamycin phosphate should be prescribed with caution in atopic individuals.
Drug Interactions
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Pregnancy: Teratogenic effects
In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters has not been associated with an increased frequency of congenital abnormalities. There are no adequate studies in pregnant women during the first trimester of pregnancy. Clindamycin should be used during the first trimester of pregnancy only if clearly needed.
Nursing Mothers
It is not known whether clindamycin is excreted in breast milk following use of clindamycin phosphate. However, orally and parentally administered clindamycin has been reported to appear in breast milk. Clindamycin has the potential to cause adverse effects on the breast-fed infant's gastrointestinal flora. Monitor the breast-fed infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breast-fed child from clindamycin or from the underlying maternal condition.
Clinical Considerations
If used during lactation and clindamycin phosphate is applied to the chest, care should be taken to avoid accidental ingestion by the infant.
Pediatric Use
Safety and effectiveness in pediatric patients under the age of 12 have not been established.
Geriatric Use
Clinical studies for clindamycin phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
ADVERSE REACTIONS
In 18 clinical studies of various formulations of clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent adverse dermatologic events [see table below].
Number of Patients Reporting Events # not recorded
* of 126 subjects
Treatment Emergent Adverse Event
Solution
n=553(%)
Gel
n=148(%)
Lotion
n=160(%)
Burning
62 (11)
15 (10)
17 (11)
Itching
36 (7)
15 (10)
17 (11)
Burning/Itching
60 (11)
# ( – )
# ( – )
Dryness
105 (19)
34 (23)
29 (18)
Erythema
86 (16)
10 (7)
22 (14)
Oiliness/Oily Skin
8 (1)
26 (18)
12* (10)
Peeling
61 (11)
# ( – )
11 (7)
Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally.
Cases of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis) have been reported as adverse reactions in patients treated with oral and parenteral formulations of clindamycin and rarely with topical clindamycin (see WARNINGS).
Abdominal pain, gastrointestinal disturbances, gram-negative folliculitis, eye pain and contact dermatitis have also been reported in association with the use of topical formulations of clindamycin.
To report SUSPECTED ADVERSE REACTIONS, contact Solaris Pharma Corporation at 1-833-919-0527 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Topically applied clindamycin phosphate can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Clindamycin phosphate gel USP, 1% containing clindamycin phosphate equivalent to 10 mg clindamycin per gram is available in the following sizes:
60 gram tube (NDC 73473-300-60)
30 gram tube (NDC 73473-300-30)
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
Protect from freezing.
Rx Only
Manufactured for:
Solaris Pharma Corporation
Bridgewater, NJ 08807Revised 03/2020 N5230
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLINDAMYCIN PHOSPHATE
clindamycin phosphate gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73473-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 10 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73473-300-60 1 in 1 CARTON 01/03/2025 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:73473-300-30 1 in 1 CARTON 01/03/2025 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211872 01/03/2025 Labeler - Solaris Pharma Corporation (079904672)


