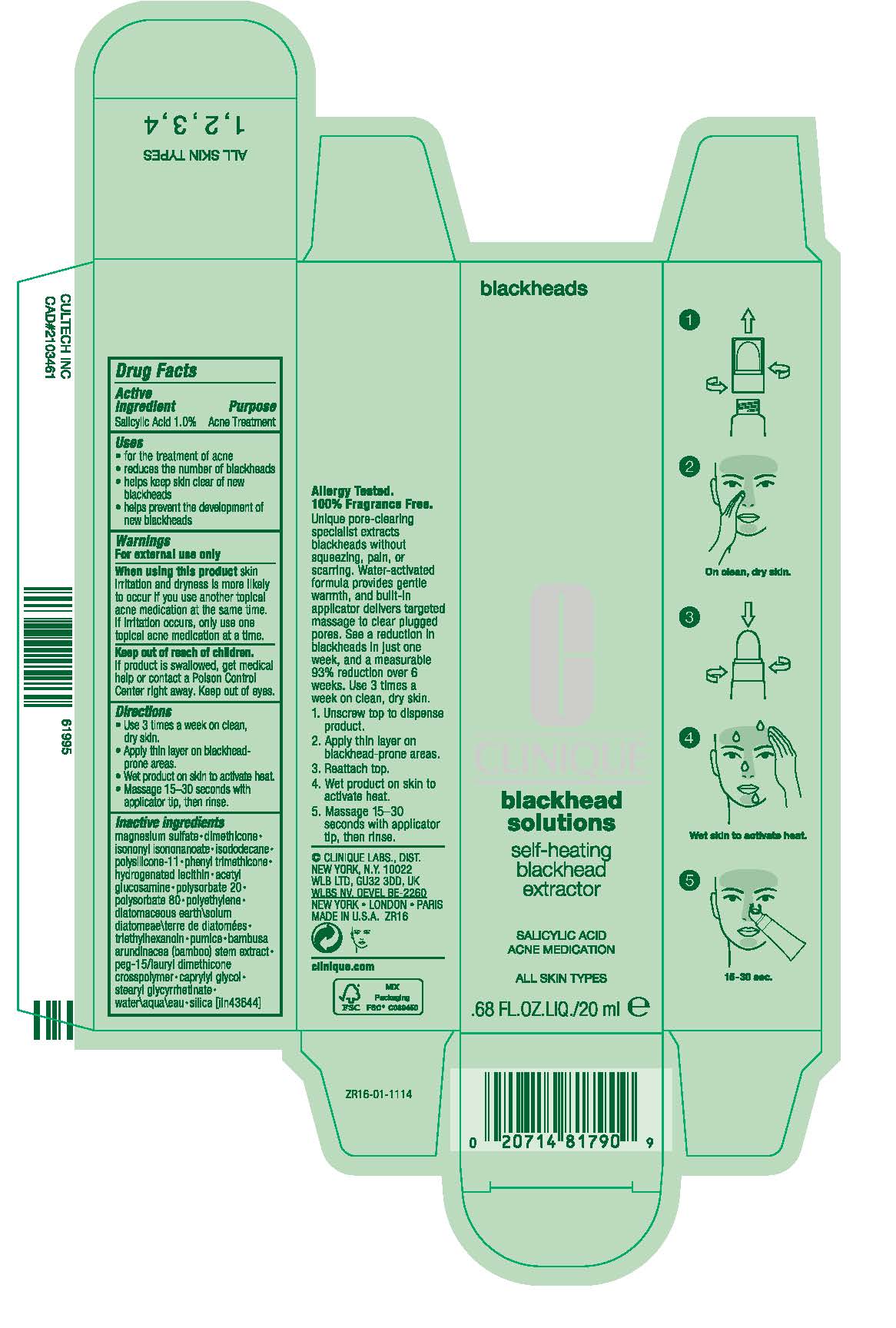
Label: BLACKHEAD SOLUTIONS SELF-HEATING BLACKHEAD EXTRACTOR ACNE MEDICATION- salicylic acid cream
- NDC Code(s): 49527-061-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
magnesium sulfate • dimethicone • isononyl isononanoate • isododecane • polysilicone-11 • phenyl trimethicone • hydrogenated lecithin • acetyl glucosamine • polysorbate 20 • polysorbate 80 • polyethylene • diatomaceous earth\solum diatomeae\terre de diatomées • triethylhexanoin • pumice • bambusa arundinacea (bamboo) stem extract • peg-15/lauryl dimethicone crosspolymer • caprylyl glycol • stearyl glycyrrhetinate • water\aqua\eau • silica [iln43644]
- PRINCIPAL DISPLAY PANEL - 20 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
BLACKHEAD SOLUTIONS SELF-HEATING BLACKHEAD EXTRACTOR ACNE MEDICATION
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) BAMBUSA BAMBOS STEM (UNII: NRA4497HC5) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) DIMETHICONE (UNII: 92RU3N3Y1O) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) N-ACETYLGLUCOSAMINE (UNII: V956696549) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) PUMICE (UNII: NT5NN5KL16) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-061-01 1 in 1 CARTON 08/30/2018 1 20 mL in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/30/2018 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations PALC 078364654 pack(49527-061) , label(49527-061) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(49527-061) , pack(49527-061) , label(49527-061) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-061) , pack(49527-061) , label(49527-061) Establishment Name Address ID/FEI Business Operations Northtec LLC 943871157 label(49527-061) , pack(49527-061)