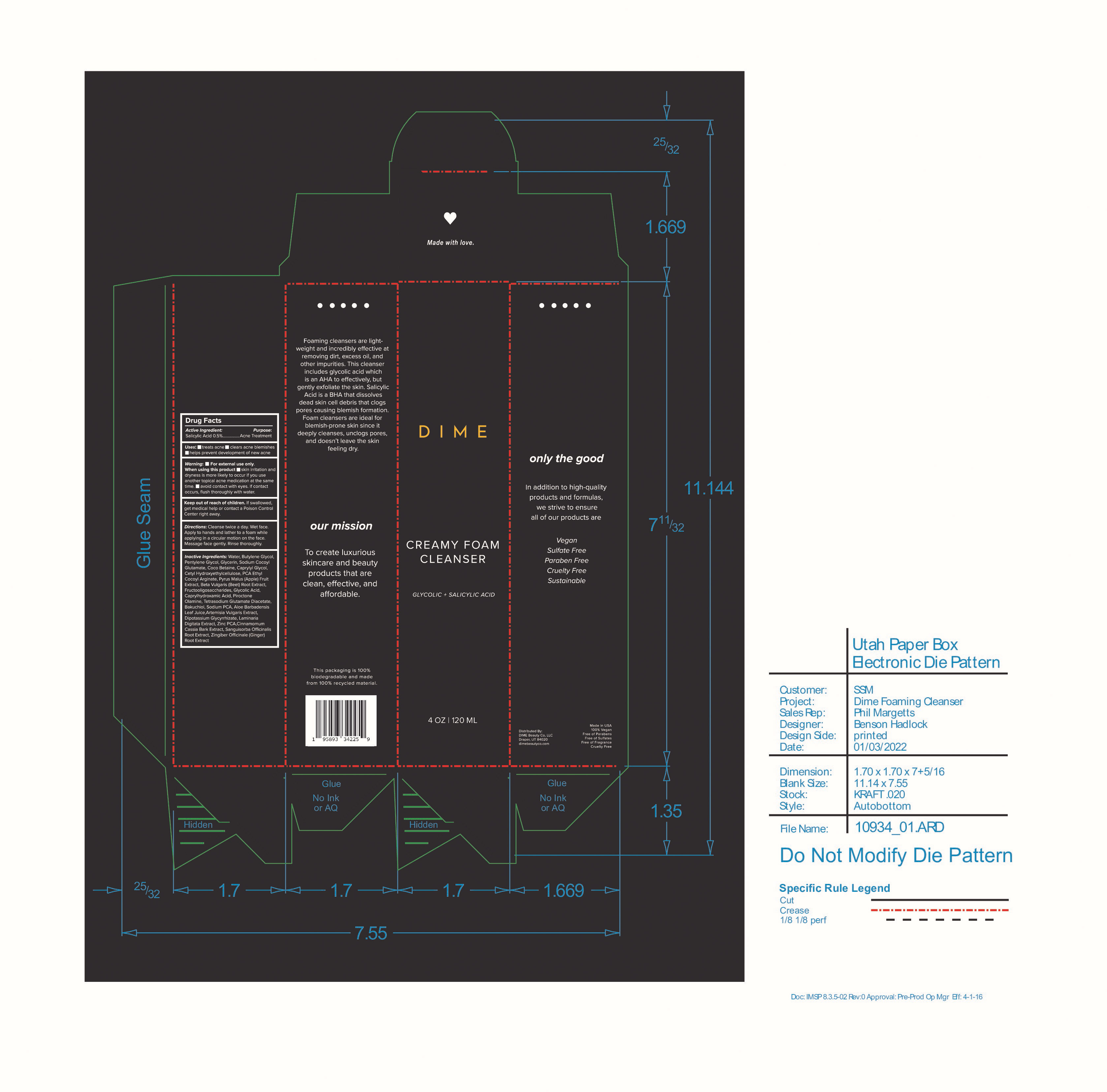
Label: CREAMY FOAM CLEANSER- salicylic acid cream
- NDC Code(s): 82870-002-01
- Packager: Dime Beauty Co, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients: Water, Butylene Glycol, Pentylene Glycol, Glycerin, Sodium Cocoyl Glutamate, Coco Betaine, Caprylyl Glycol, Cetyl Hydroxyethylcellulose, PCA Ethyl Cocoyl Arginate, Pyrus Malus (Apple) Fruit Extract, Beta Vulgaris (Beet) Root Extract, Fructooligosaccharides, Glycolic Acid, Caprylhydroxamic Acid, Piroctone Olamine, Tetrasodium Glutamate Diacetate, Bakuchiol, Sodium PCA, Aloe Barbadensis Leaf Juice, Artemisia Vulgaris Extract, Dipotassium Glycyrrhizate, Laminaria Digitata Extract, Zinc PCA, Cinnamomum Cassia Bark Extract, Sanguisorba Officinalis Root Extract, Zingiber Officinale (Ginger) Root Extract
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CREAMY FOAM CLEANSER
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82870-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CETYL HYDROXYETHYLCELLULOSE (350000 MW) (UNII: T7SWE4S2TT) GLYCOLIC ACID (UNII: 0WT12SX38S) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) BEET (UNII: N487KM8COK) WATER (UNII: 059QF0KO0R) SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) PYRROLIDONE CARBOXYLIC ACID ETHYL COCOYL ARGINATE (UNII: A53122S09O) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) COCO-BETAINE (UNII: 03DH2IZ3FY) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) APPLE (UNII: B423VGH5S9) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHINESE CINNAMON (UNII: WS4CQ062KM) BAKUCHIOL (UNII: OT12HJU3AR) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) LAMINARIA DIGITATA (UNII: 15E7C67EE8) ZINC PIDOLATE (UNII: C32PQ86DH4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82870-002-01 120 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/11/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/11/2022 Labeler - Dime Beauty Co, LLC (058459261)