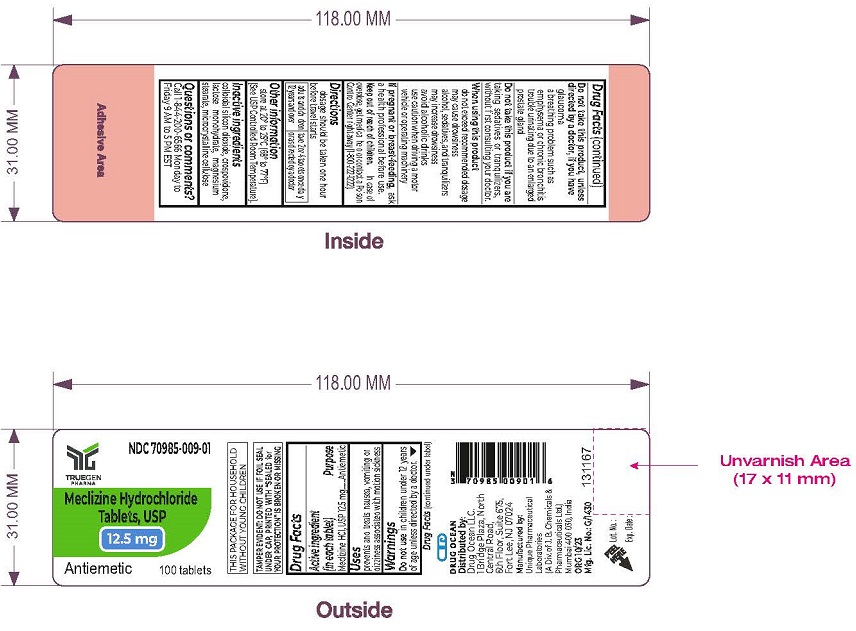
Label: MECLIZINE HCL- meclizine hydrochloride tablet
- NDC Code(s): 70985-009-01
- Packager: Drug Ocean LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in each tablet)
- Purpose
- Uses:
- Warnings:
- Do not use in children under 12 years of age unless directed by a doctor.
- ASK DOCTOR/PHARMACIST
- When using product
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
- Directions
- Other Information
- Inactive ingredients
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
TAMPER EVIDENT: DO NOT USE IF FOIL SEAL UNDER CAP, PRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING
Distributed by:
Drug Ocean LLC,
1 Bridge Plaza, North Central Road,6th Floor, Suite 675,
Fort Lee, NJ 07024
Manufactured by:
Unique Pharmaceutical Laboratories
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd.)
Mumbai 400 030, IndiaORG 12/23
- PRINCIPAL DISPLAY PANEL - 12.5 mg Tablet Label
-
INGREDIENTS AND APPEARANCE
MECLIZINE HCL
meclizine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70985-009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 12.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white (White to Off White) Score no score Shape ROUND Size 7mm Flavor Imprint Code AB;12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70985-009-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/25/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 06/25/2021 Labeler - Drug Ocean LLC (080381835) Registrant - Drug Ocean LLC (080381835) Establishment Name Address ID/FEI Business Operations Unique Pharmaceutical Laboratories 650434645 manufacture(70985-009)