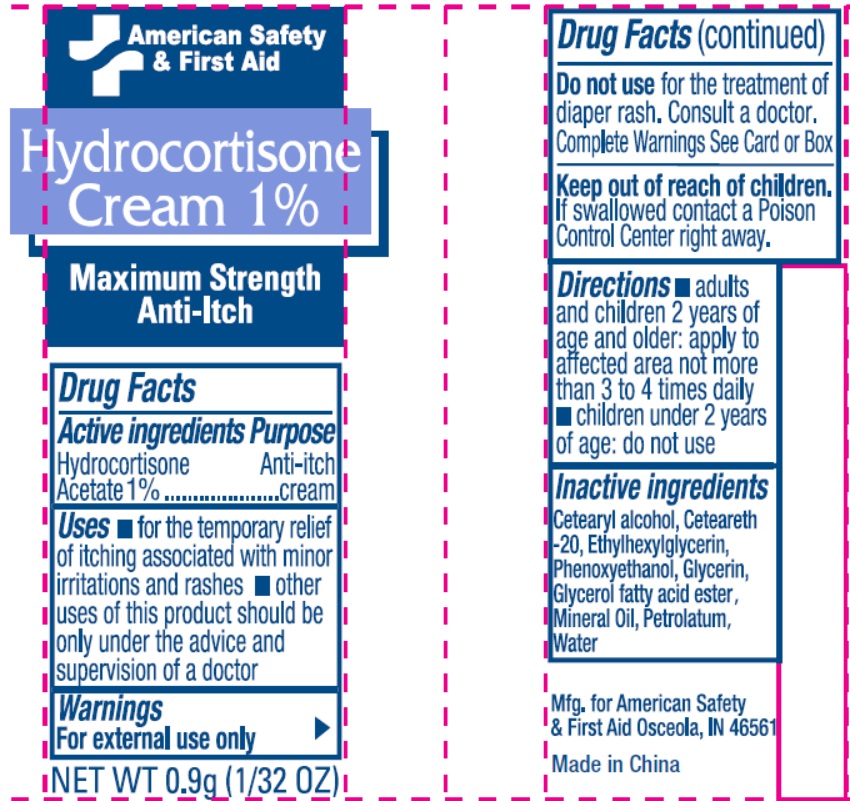
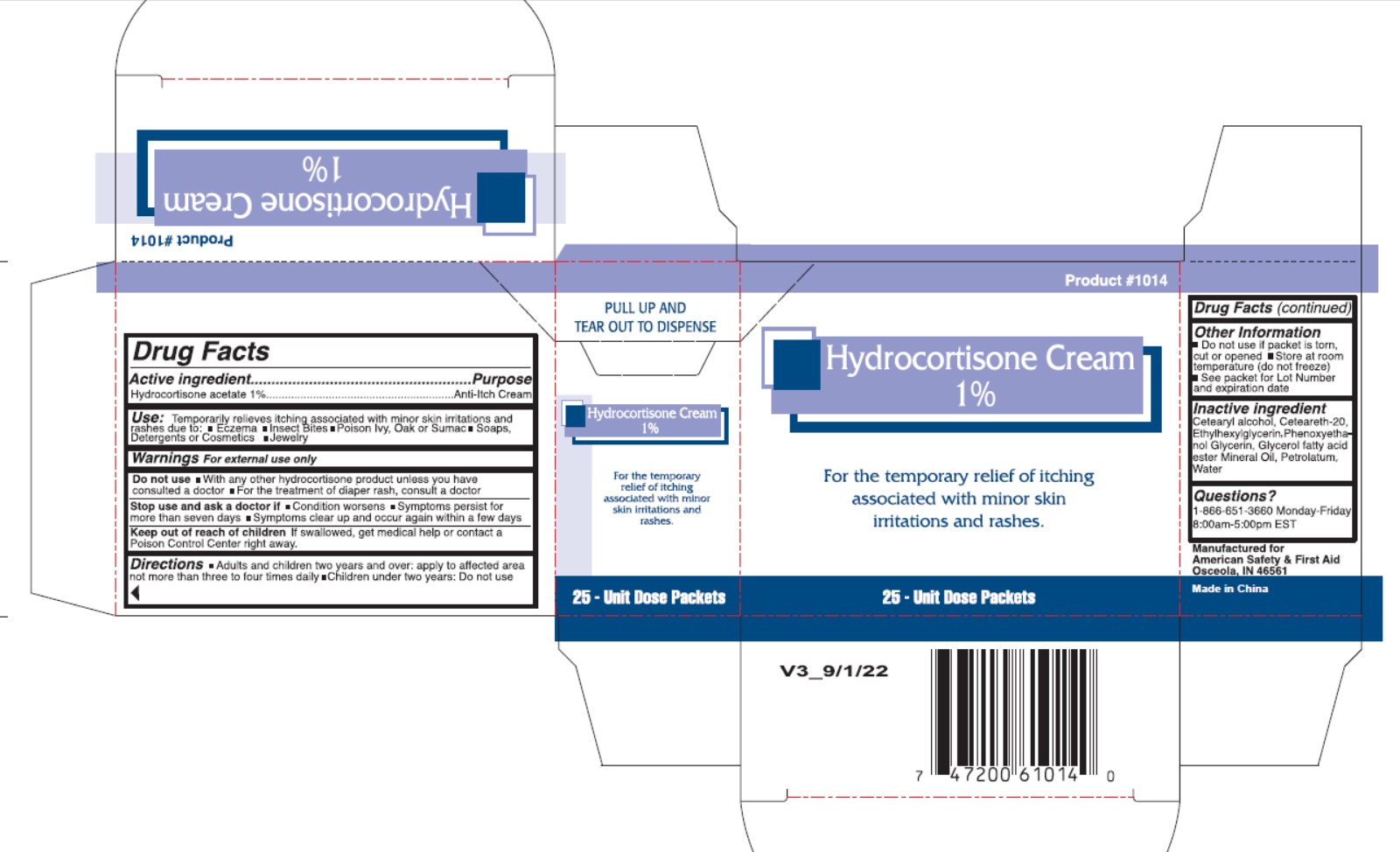
Label: AMERICAN SAFETY AND FIRST AID- hydrocortisone acetate cream
- NDC Code(s): 71927-015-01, 71927-015-02
- Packager: Orazen Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
- Directions
- Inactive Ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
AMERICAN SAFETY AND FIRST AID
hydrocortisone acetate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71927-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71927-015-02 25 in 1 BOX 10/17/2022 1 NDC:71927-015-01 0.9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/17/2022 Labeler - Orazen Inc (080916640)