Label: PROGESTERONE capsule
- NDC Code(s): 76420-072-10, 76420-072-30, 76420-072-90, 76420-073-10, view more
- Packager: Asclemed USA, Inc.
- This is a repackaged label.
- Source NDC Code(s): 59651-152, 59651-153
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER and PROBABLE DEMENTIA FOR ESTROGEN PLUS PROGESTIN THERAPY
Cardiovascular Disorders and Probable Dementia
Estrogens plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders and Probable dementia.)
The Women’s Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of deep vein thrombosis, pulmonary embolism, stroke and myocardial infarction in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders.)
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. (See CLINICAL STUDIES and WARNINGS, Probable dementia and PRECAUTIONS, Geriatric Use.)
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer. (See CLINICAL STUDIES and WARNINGS, Malignant neoplasms, Breast Cancer.)
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins.
Progestins with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Close -
DESCRIPTIONProgesterone capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C - 21H - 30O ...
-
CLINICAL PHARMACOLOGYProgesterone capsules are an oral dosage form of micronized progesterone which is chemically identical to progesterone of ovarian origin. The oral bioavailability of progesterone is increased ...
-
INDICATIONS AND USAGEProgesterone capsules are indicated for use in the prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving conjugated estrogens tablets. They are ...
-
CONTRAINDICATIONSProgesterone capsules should not be used in women with any of the following conditions: Progesterone capsules should not be used in patients with known hypersensitivity to its ingredients ...
-
WARNINGSSee - BOXED WARNING. 1. Cardiovascular disorders - An increased risk of pulmonary embolism, deep vein thrombosis (DVT), stroke, and myocardial infarction has been ...
-
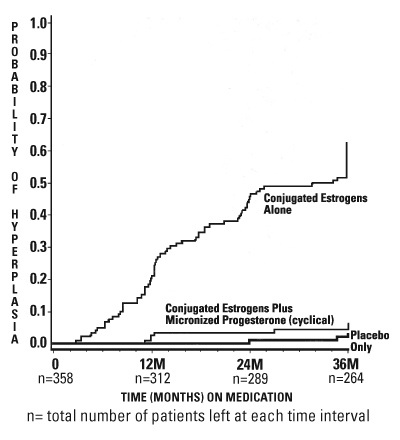
PRECAUTIONSA. General - 1. Addition of a progestin when a woman has not had a hysterectomy - Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or ...
-
ADVERSE REACTIONSSee BOXED WARNING, WARNINGS and - PRECAUTIONS. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in ...
-
OVERDOSAGENo studies on overdosage have been conducted in humans. In the case of overdosage, progesterone capsules should be discontinued and the patient should be treated symptomatically.
-
DOSAGE AND ADMINISTRATIONPrevention of Endometrial Hyperplasia - Progesterone capsules should be given as a single daily dose at bedtime, 200 mg orally for 12 days sequentially per 28-day cycle, to a postmenopausal ...
-
HOW SUPPLIEDProgesterone Capsules 100 mg are white to off white, round shaped soft gelatin capsules imprinted with “P1” using black ink and containing white to off white suspension. Bottles of ...
-
PATIENT INFORMATIONProgesterone Capsules 100 mg and 200 mg - (proe jes' ter one) Read this PATIENT INFORMATION before you start taking progesterone capsules and read what you get each ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mgProgesterone Capsules 100 mg - DO NOT USE IF ALLERGIC TO PEANUTS - Rx only
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mgProgesterone Capsules 200 mg - DO NOT USE IF ALLERGIC TO PEANUTS - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information