Label: ACNE CLEANSER- salycilic acid gel
- NDC Code(s): 78518-011-01
- Packager: MONAT GLOBAL CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only
- Directions
-
INACTIVE INGREDIENT
Inactive Ingredients
Water, Sodium Cocoyl Glutamate, Propanediol, Sorbeth-230 Tetraoleate, Cocamidopropyl Betaine, Sodium C14-16 Olefin Sulfonate, Sodium Cocoyl Isethionate, PEG-120 Methyl Glucose Dioleate, Niacinamide, Cocamide MIPA, Glycerin, Coconut Acid, Sodium Isethionate, Sodium PCA, Maclura Cochinchinensis Leaf Prenylflavonoids, Glycol Distearate, Laureth-4, Sodium Chloride, Sodium Benzoate, Potassium Sorbate, Polyquaternium-10, Decyl Glucoside, Sorbitan Laurate, Isopropyl Alcohol.
- Questions or Comments
- PRIMARY PACKAGE
- OUTER PACKAGE
-
INGREDIENTS AND APPEARANCE
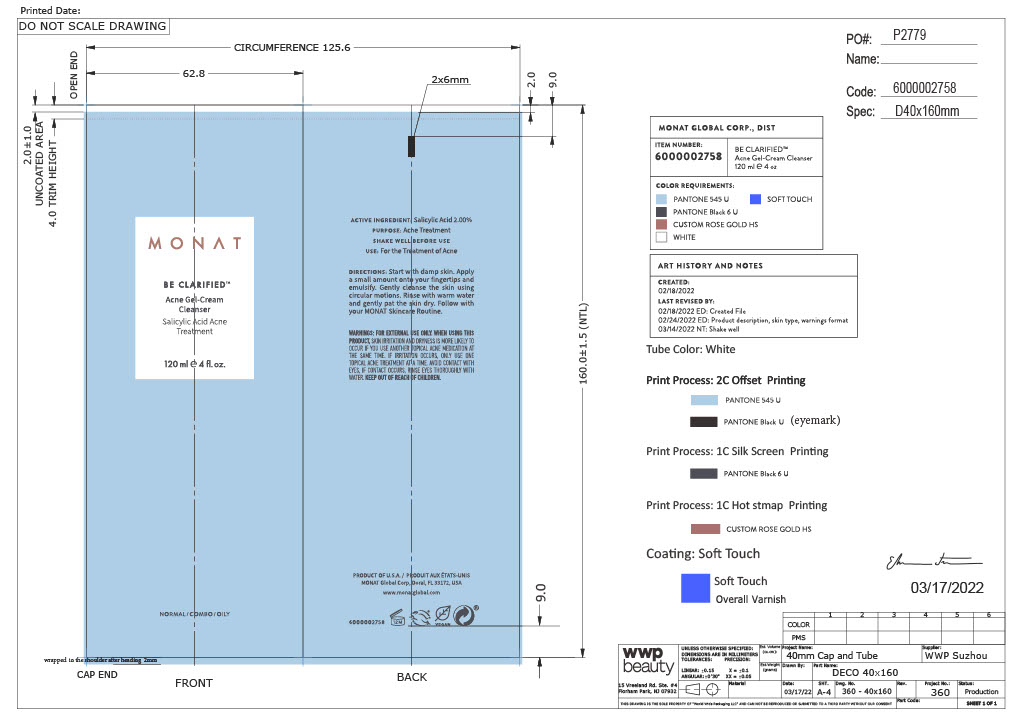
ACNE CLEANSER
salycilic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78518-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 100 mg Inactive Ingredients Ingredient Name Strength LAURETH-4 (UNII: 6HQ855798J) SODIUM ISETHIONATE (UNII: 3R36J71C17) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) PROPANEDIOL (UNII: 5965N8W85T) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCOL DISTEARATE (UNII: 13W7MDN21W) DECYL GLUCOSIDE, .ALPHA.- (UNII: 47YY6O286H) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) COCONUT ACID (UNII: 40U37V505D) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) POLYQUATERNIUM-10 (400 CPS AT 2%) (UNII: HB1401PQFS) SODIUM CHLORIDE (UNII: 451W47IQ8X) MACLURA COCHINCHINENSIS WHOLE (UNII: X083FY34PH) ISOPROPYL ALCOHOL (UNII: ND2M416302) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78518-011-01 1 in 1 CARTON 04/04/2023 1 120 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 04/04/2023 Labeler - MONAT GLOBAL CORP (027036949) Establishment Name Address ID/FEI Business Operations Oxygen Development 137098492 manufacture(78518-011)



 SECONDARY-OUTER PACKAGE
SECONDARY-OUTER PACKAGE