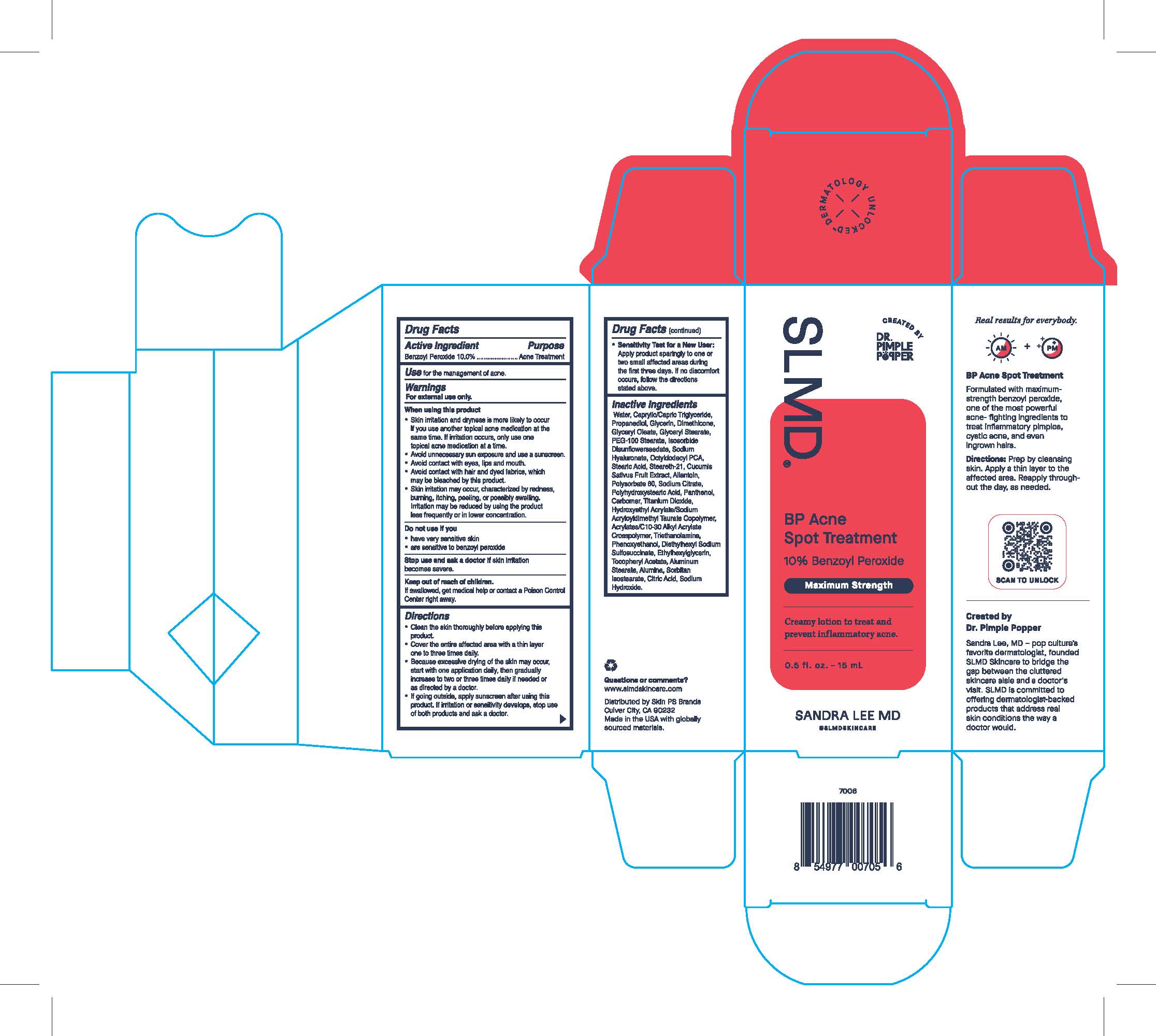
Label: BP ACNE SPOT TREATMENT- acne spot treatment lotion
- NDC Code(s): 73318-7002-5
- Packager: Skin PS Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
-
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen.
- avoid contact with eyes, lips and mouth.
- avoid contact with hair and dyed fabrics, which may be bleached by this product.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
- Do not use if you
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three daily if needed or as directed by a doctor.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Sensitivity Test for a New User:
- Apply product sparingly to one or two small affected areas during the first three days. if no discomfort occurs, follow the directions stated above.
-
Inactive Ingredients
Water, Caprylic/Capric Triglyceride, Propanediol, Glycerin, Dimethicone, Glyceryl Oleate, Glyceryl Stearate, PEG-100 Stearate, Isosorbide Disunflowerseedate, Sodium Hyaluronate, Octyldodecyl PCA, Stearic Acid, Steareth-21, Cucumis Sativus Fruit Extract, Allantoin, Polysorbate 60, Sodium Citrate, Polyhydroxystearic Acid, Panethol, Carbomer, Titanium Dioxide, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Phenoxyethanol, Diethylhexyl Sodium Sulfosuccinate, Ethylhexylglycerin, Tocopheryl Acetate, Aluminum Stearate, Alumina, Sorbitan Isostearate, Citric Acid, Sodium Hydroxide.
- Questions or comments?
-
SLMD
Sandra Lee MD
Skin Care by Dr. Pimple Popper
BP Acne Spot Treatment
10% Benzoyl Peroxide
Maximum Strength
Creamy lotion to treat and prevent inflammatory acne.
0.5 fl. oz. - 15 mL
SANDRA LEE MD
@SLMDSKINCARE
Unit Carton:
Real results for everybody.
AM + PM
BP Acne Spot Treatment
Formulated with maximum-strength benzoyl peroxide, one of the mose powerful acne-fighting ingredients to treat inflammatory pimples, cystic acne, and even ingrown hairs.
Directions: Prep by cleansing skin. Apply a thin layer to the affected area. Reapply throughout the day, as needed.
Created by Dr. Pimple Popper
Sandra Lee, MD - pop culture's favorite dermatologist, founded SLMD Skincare to bridge the gap between the cluttered skincare aisle and a doctor's visit. SLMD is committed to offering dermatologist-backed products that address real skin conditions the way a doctor would.
-
INGREDIENTS AND APPEARANCE
BP ACNE SPOT TREATMENT
acne spot treatment lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73318-7002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) TROLAMINE (UNII: 9O3K93S3TK) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL OLEATE (UNII: 4PC054V79P) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-100 STEARATE (UNII: YD01N1999R) WATER (UNII: 059QF0KO0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) 2-OCTYLDODECYL 5-OXO-L-PROLINATE (UNII: E25TY46YTD) STEARIC ACID (UNII: 4ELV7Z65AP) STEARETH-21 (UNII: 53J3F32P58) CUCUMBER (UNII: YY7C30VXJT) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALUMINUM OXIDE (UNII: LMI26O6933) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PANTHENOL (UNII: WV9CM0O67Z) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM CITRATE (UNII: 1Q73Q2JULR) DOCUSATE SODIUM (UNII: F05Q2T2JA0) ALUMINUM STEARATE (UNII: U6XF9NP8HM) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) DIMETHICONE (UNII: 92RU3N3Y1O) ALLANTOIN (UNII: 344S277G0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73318-7002-5 1 in 1 CARTON 06/22/2023 1 15 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/22/2023 Labeler - Skin PS Brands (081085221) Registrant - Skin PS Brands (081085221) Establishment Name Address ID/FEI Business Operations Owen Biosciences 790003045 manufacture(73318-7002)

