Label: BISACODYL tablet, delayed release
- NDC Code(s): 0904-6748-17, 0904-6748-60, 0904-6748-80
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- it may cause stomach discomfort, faintness, and cramps
- stomach pain, nausea or vomiting
- Directions
- Other information
-
Inactive ingredients
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, lactose anhydrous, magnesium stearate, methylparaben, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparaben, shellac glaze, simethicone, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
- Questions or comments?
-
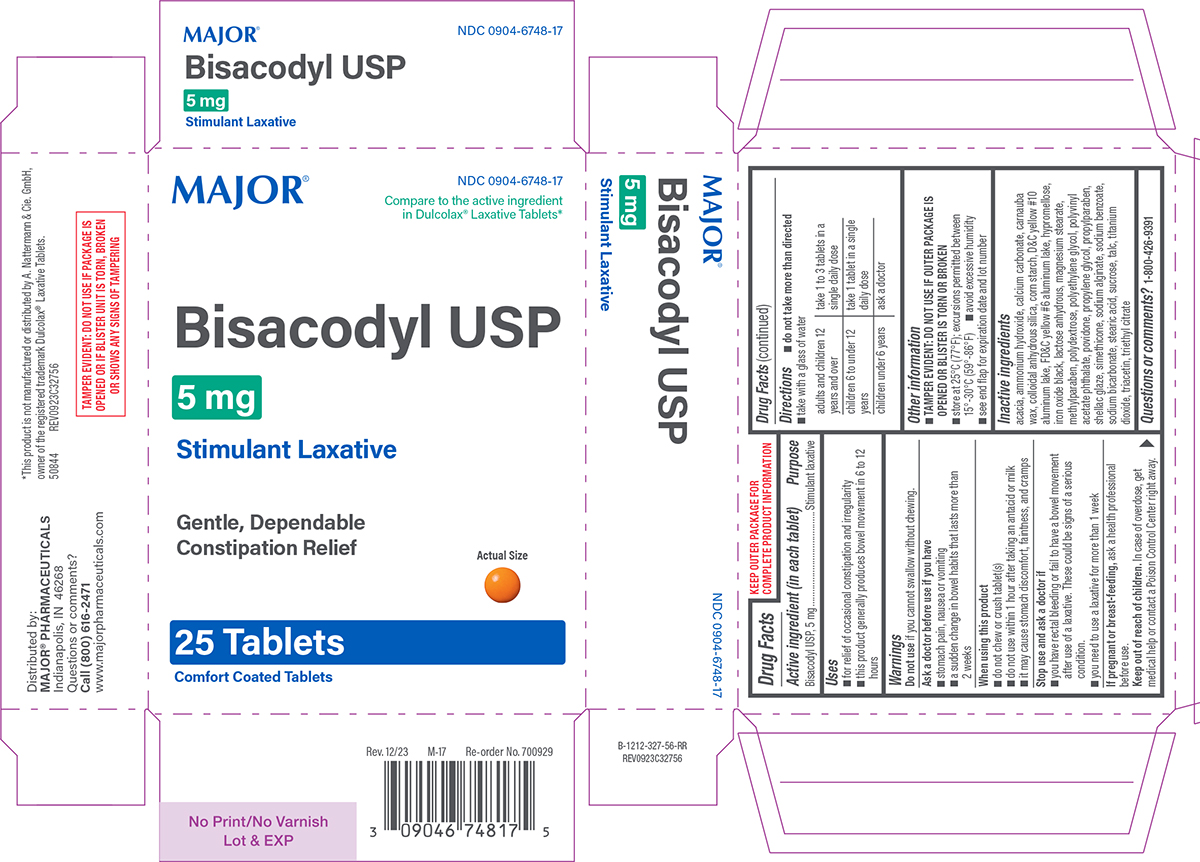
Principal display panel
NDC 0904-6748-17
Compare to the active ingredient
in Dulcolax® Laxative Tablets*Major®
Bisacodyl USP
5 mgStimulant Laxative
Gentle, Dependable
Constipation ReliefActual Size
25 TABLETS
Comfort Coated TabletsTAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING*This product is not manufactured or distributed by A. Nattermann & Cie. GmbH,
owner of the registered trademark Dulcolax® Laxative Tablets.
50844 REV0923C32756Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
Questions or comments?
Call (800) 616-2471
www.majorpharmaceuticals.comM-17 Rev. 12/23 Re-order No. 700929
Major 44-327
-
INGREDIENTS AND APPEARANCE
BISACODYL
bisacodyl tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6748 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 5 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) AMMONIA (UNII: 5138Q19F1X) CALCIUM CARBONATE (UNII: H0G9379FGK) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Polyvinyl Acetate Phthalate (UNII: 58QVG85GW3) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) SODIUM ALGINATE (UNII: C269C4G2ZQ) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color orange Score no score Shape ROUND Size 6mm Flavor Imprint Code 5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6748-17 1 in 1 CARTON 12/01/2018 1 25 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:0904-6748-60 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/01/2018 3 NDC:0904-6748-80 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/01/2018 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 manufacture(0904-6748) , pack(0904-6748) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0904-6748) , pack(0904-6748) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(0904-6748) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0904-6748) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0904-6748)

